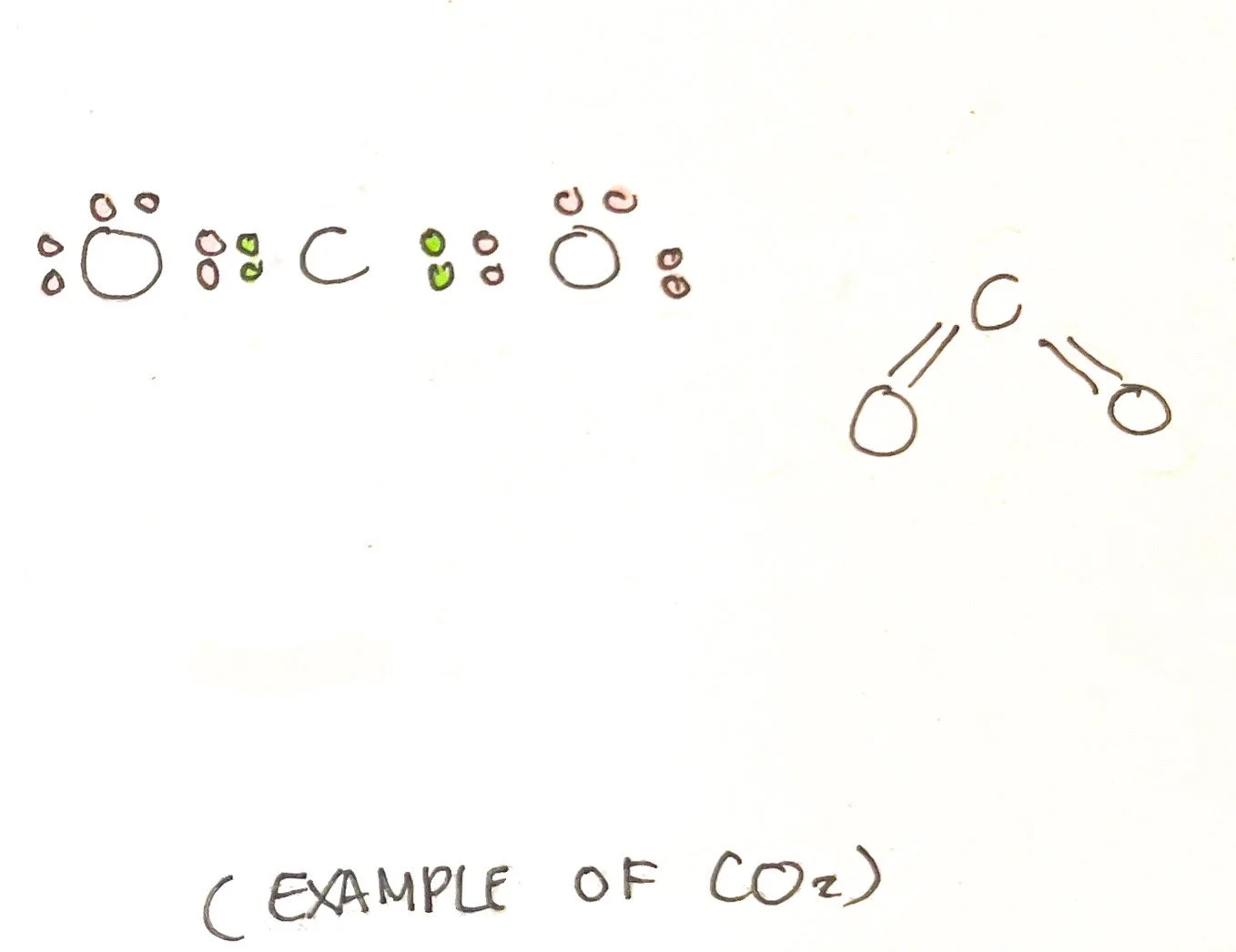Atoms, Elements,& Molecules
You should be able to:
Identify the types and functions of atoms, elements, and molecules.
Learn about the types of reactions that occur within the body.
Understand the differences between molecular, condensed, and structural formulas and the rules that define them.
Explore the different types of functional groups found within molecules.
Learning the basics of organic chemistry (the study of organic matter containing carbon) can improve understanding of all life and life processes. It plays a role within all components of the human body and nutrients that keep us alive. This guide intends to provide a comprehensive understanding of organic chemistry as it relates to the body and nutrition.
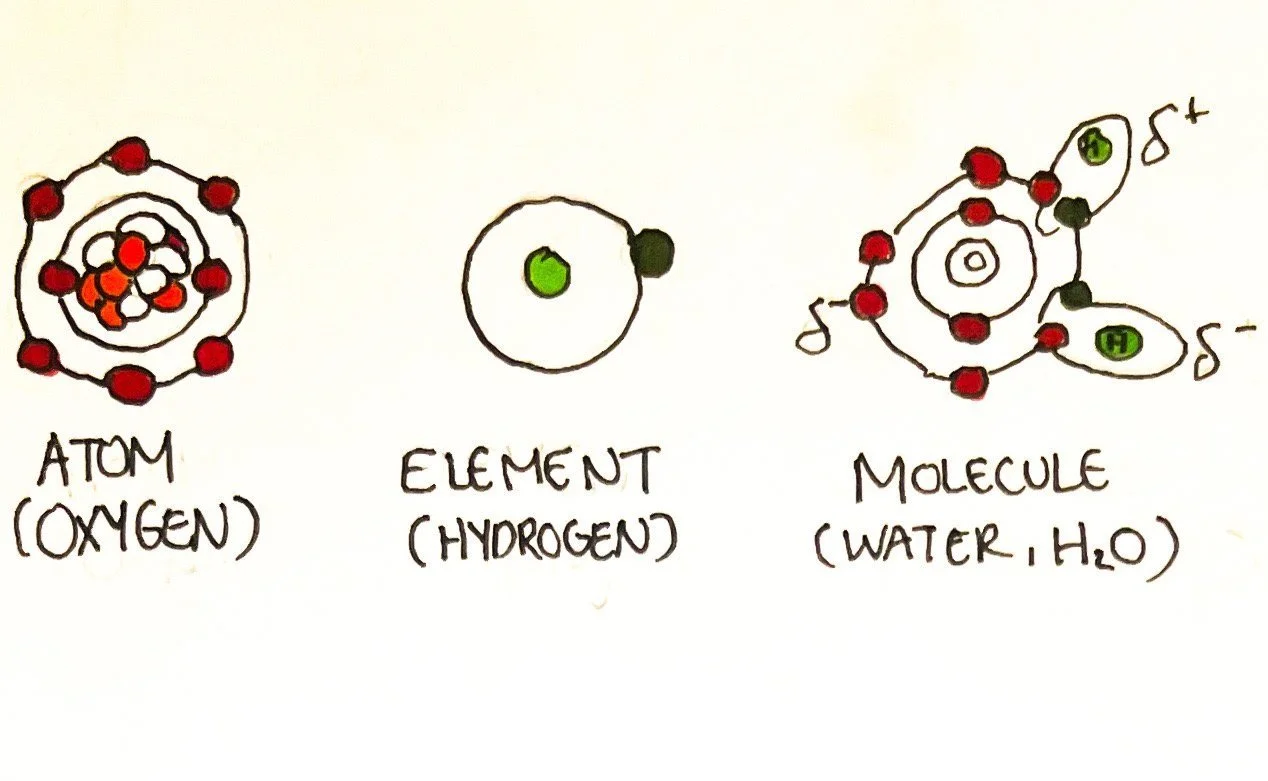
What are atoms, elements, and molecules?
Atoms are defined as the smallest unit of matter. Matter is anything that takes up space and has mass (or an amount of any particular component). Elements are composed of atoms but cannot be broken down any further. Elements are found among living species and categorized as major, lesser, and trace. Molecules are when two or more atoms bond and exist at many levels among living things.
What to Know About Elements
Elements can be divided into major, lesser, and trace categories.¹ Major elements contribute 96% of body mass while lesser elements represent approximately 3.6%.¹ Trace elements, namely 14, account for the remaining .4%.¹ Here are descriptions of some major and lesser elements:
Major Elements*
Carbon - found within nutrients carbohydrates, lipids, and protein. DNA is also made up of carbon.
Oxygen - found within water and used during ATP synthesis.
Hydrogen - found within water and nutrient structures.
Nitrogen - found within protein structures.
Lesser Elements*
Sodium - found within extracellular fluid (fluid outside the cell) in cation form, generates nerve signals.¹
Potassium - found within intracellular fluid (fluid inside the cell) in cation form, generates nerve signals.¹
Calcium - found within bones and teeth and functions in blood clotting, muscle contraction, etc.¹
Phosphorus - found within the skeletal system and teeth and ATP structure.
Magnesium - component of enzymes and involved in nerve signals.
Sulfur - involved in nutrient metabolism and free radical elimination.¹
Chlorine - found in extracellular fluid as an anion bound to sodium maintaining fluid balance.¹
Iron - found in hemoglobin and enzyme structures.
Trace Elements - 14 trace elements are found within the body: aluminum, silicon, boron, manganese, chromium, cobalt, copper, iodine, fluorine, molybdenum, selenium, vanadium, zinc, and tin.¹
*All structures and functions not listed. All lesser elements and some trace elements listed are considered minerals found within the body.
What to Know About Atoms
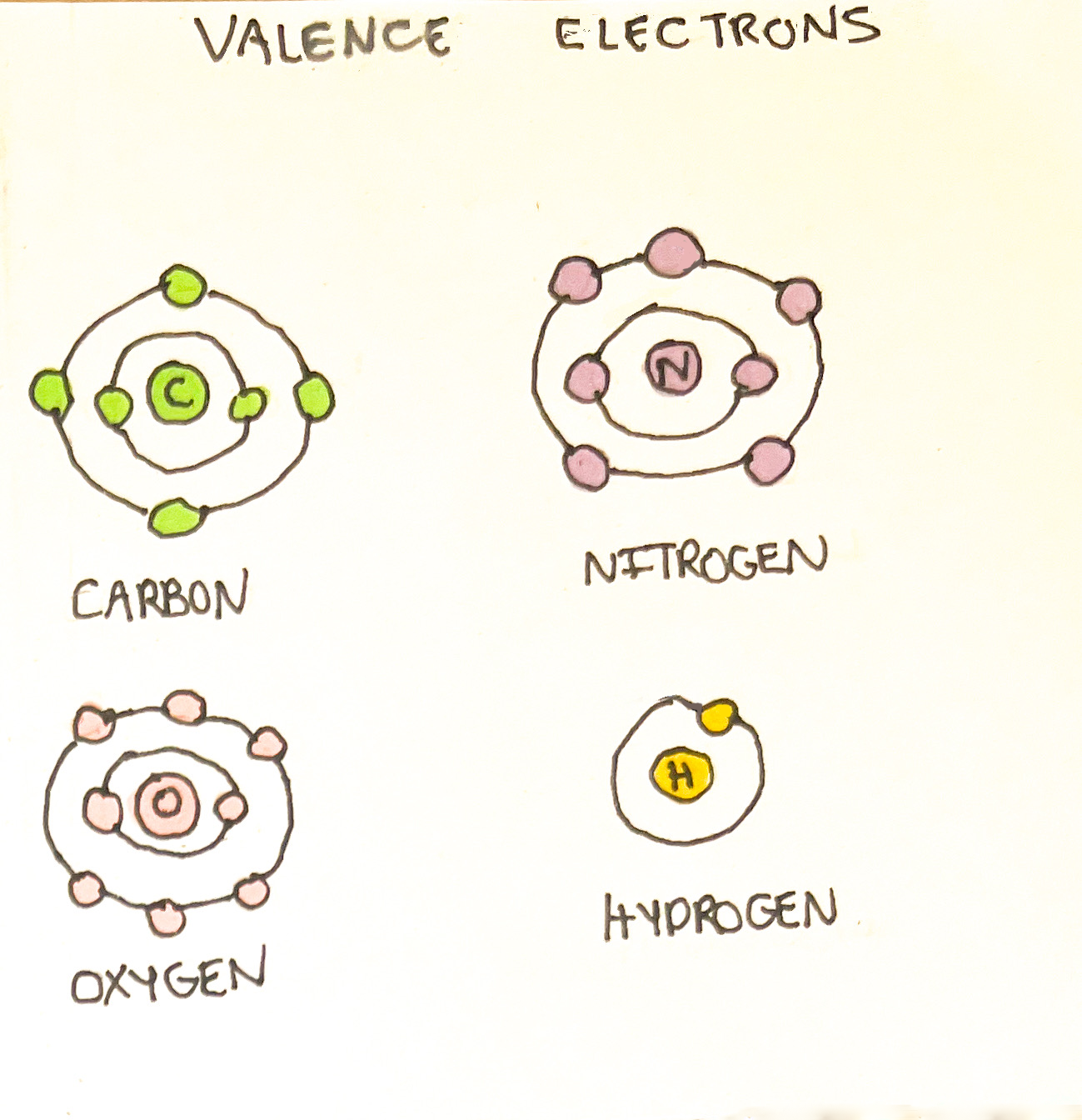
Atoms are composed of protons, neutrons, electrons, and a nucleus. The nucleus of an atom is located in the center and consists of positively charged protons and neutral (no charge) neutrons. The negatively charged electrons are located outside of the nucleus aligned in rings known as electron shells. The electron shells fill up with electrons in a particular order. The electron shell closest to the nucleus can contain up to two electrons. The second ring holds a maximum of eight; the third ring holds a maximum of 18, etc.¹ In neutral atoms, protons always equal the number of electrons in a shell.
The valence shell is the outermost electron shell. This shell would like to contain eight electrons whether it is donated or shared.¹ Most atoms strive to achieve this configuration to satisfy the octet rule. Hydrogen only has 1 valence electron but can bind readily to other atoms.¹ It requires 2 valence electrons to reach stability - an exception from the octet rule. This is useful to know to understand the types of pairings/chemical bonds between atoms.
What to Know About Molecules
Molecules form when two or more atoms bond and can be made up of the same or different atoms/elements. A subscript in a molecular formula indicates the amount of atoms/elements. If a subscript is not present, it indicates 1 atom.
Example: H₂O (2 hydrogen atoms, 1 oxygen atom)
What are chemical bonds?
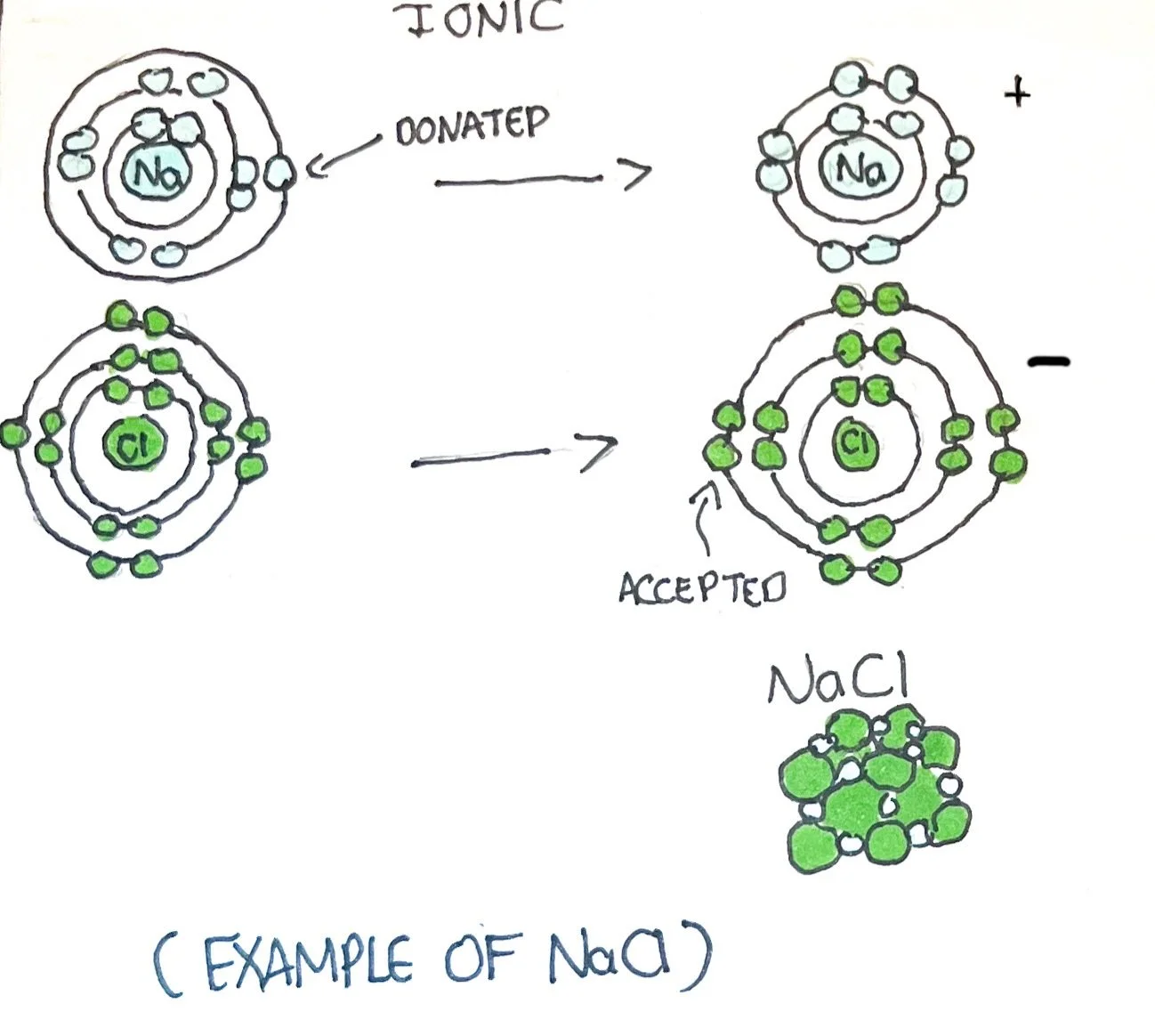
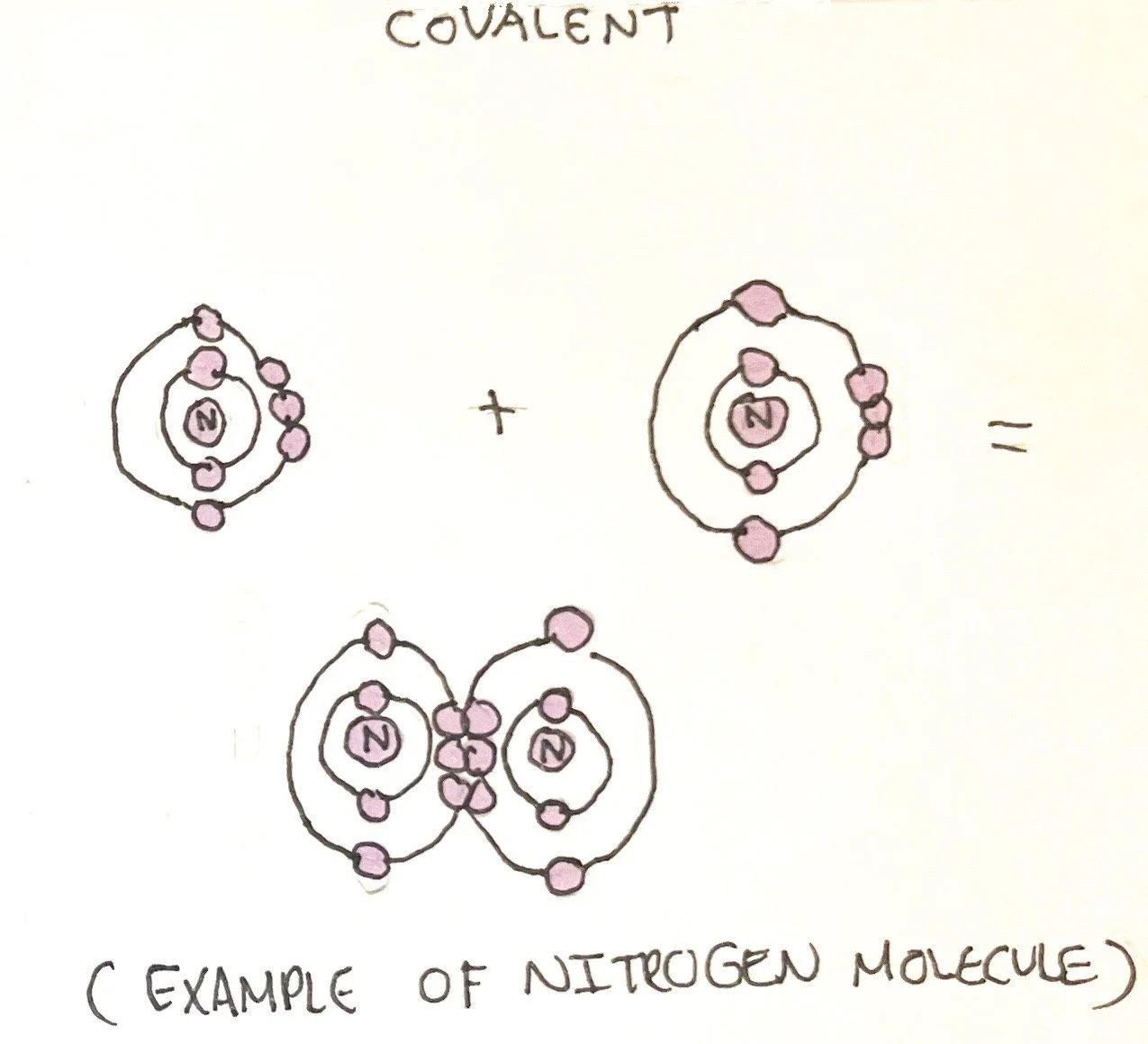
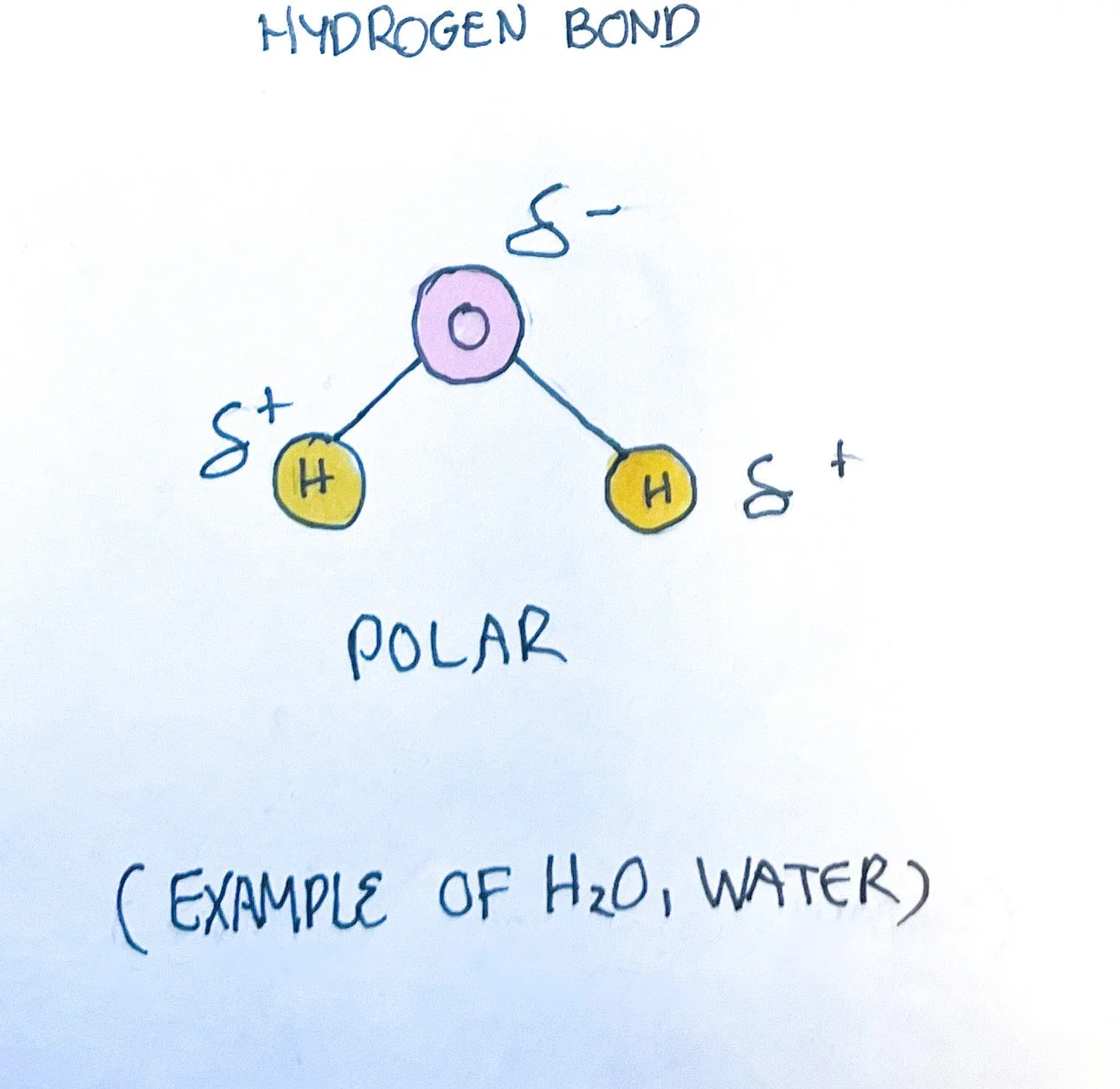
The bonding of molecules can be described as ionic, covalent, or hydrogen.
Ionic Bonds - atoms that gain or lose valence electrons become an ion. An atom with a positive charge is known as a cation, and an atom with a negative charge is an anion. The cation has a positive charge because it lost an electron (containing more protons), and the anion has a negative charge because it gained an electron (containing more electrons). A superscript of (+) or (-) indicates if the atom or molecule is a cation or anion. The number preceding the (+) or (-) is the amount gained or lost. Ions exist in multiple forms within the body and are crucial to support many reactions and metabolic processes.
Examples of ions:
Cations: Hydrogen (H⁺), sodium (Na⁺), potassium (K⁺), ammonium (NH⁴⁺), magnesium (Mg²⁺), calcium (Ca²⁺), iron (Fe³⁺)
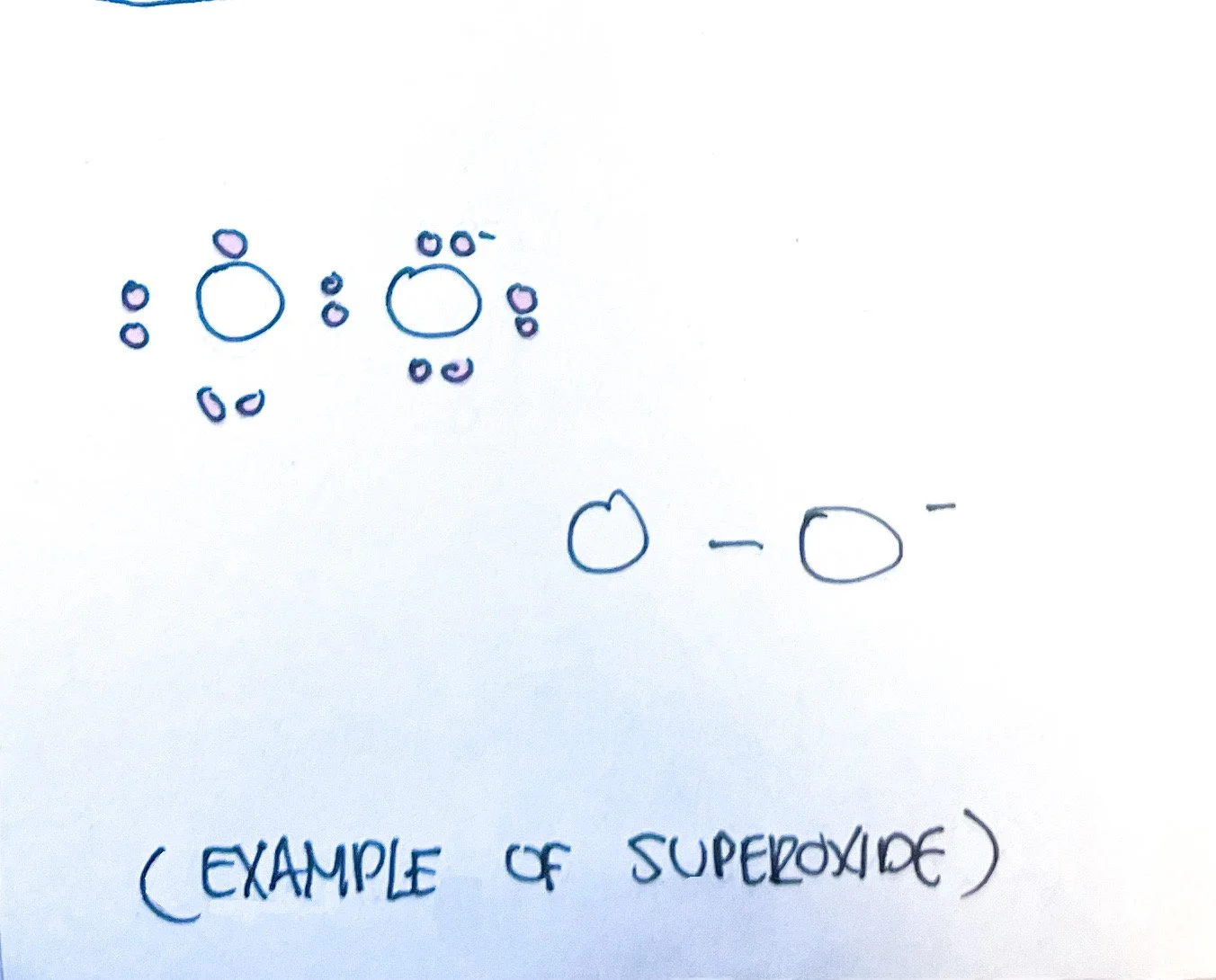
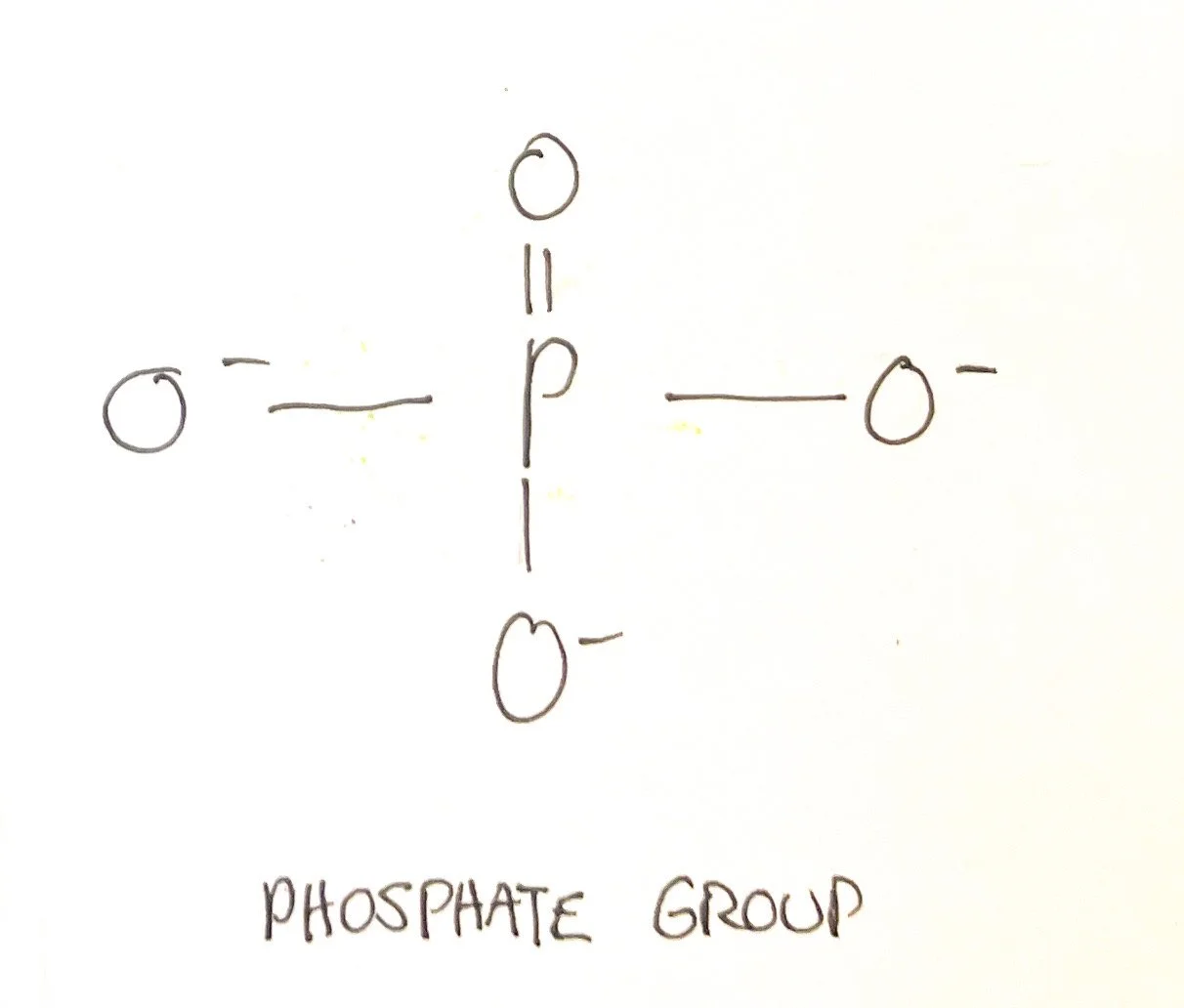
Anions: fluoride (F⁻), chloride (Cl⁻), iodide (I⁻), hydroxide (OH⁻), bicarbonate (HCO₃⁻), oxide (O²⁻), sulfate (SO₄²⁻), phosphate (PO₄³⁻)
The force of attraction between the cation and anion creates the ionic bond. The ionic bond satisfies the octet rule by containing 8 electrons within the valence shells of each atom. Breaking of the ionic bond will lead to cations and anions within body fluid - this is known as electrolytes since atoms have a positive or negative electrical charge.¹
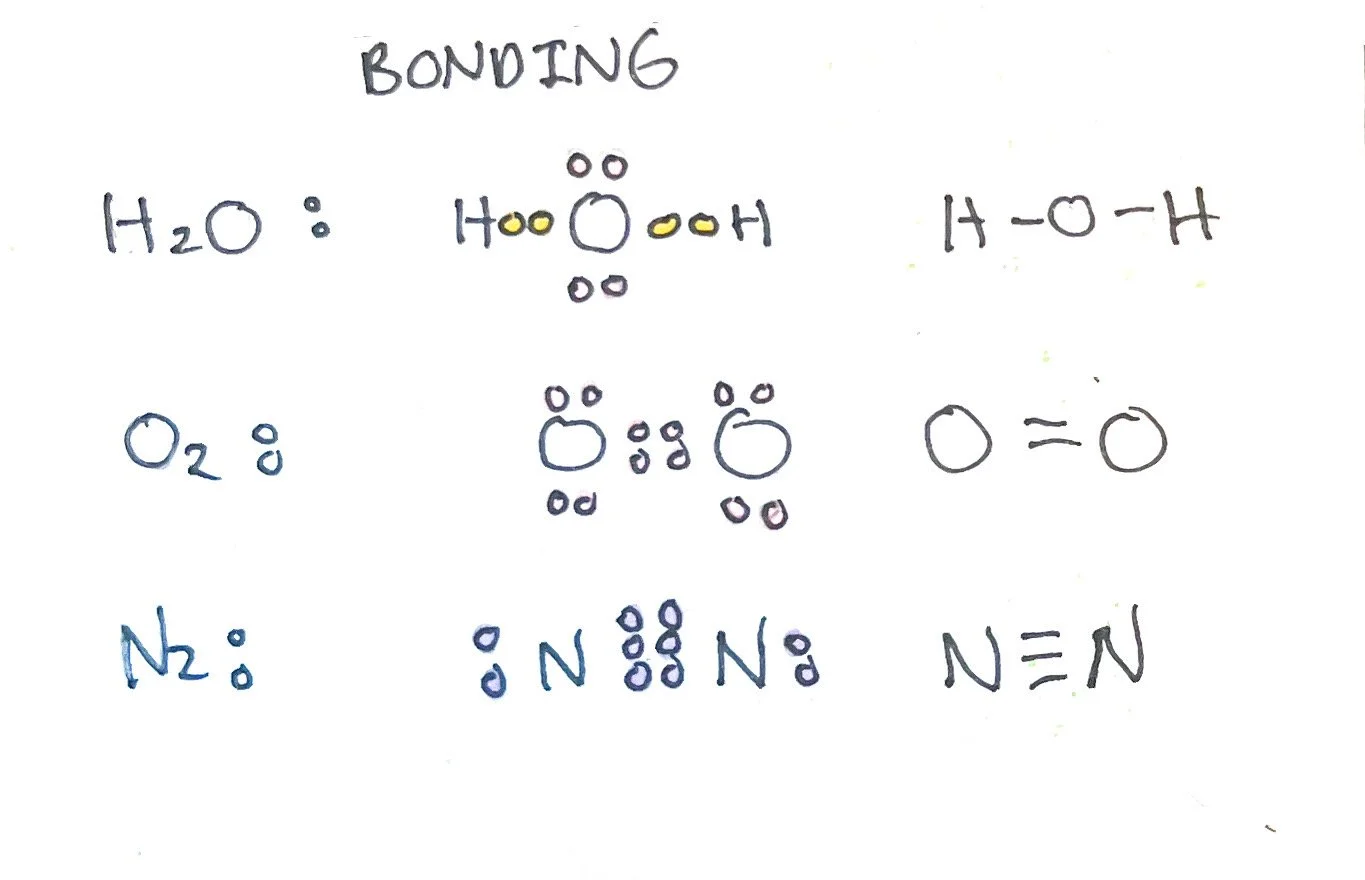
Covalent Bonds - instead of donating or losing valence electrons, atoms share valence electrons to reach an octet. A single covalent bond is defined as an electron pair shared between two atoms. Covalent bonds can also be classified as double (two pairs) and triple (3 pairs). This can happen between the same two atoms or two different atoms. The more bonds between atoms, the stronger the covalent bond is. However, it is weaker than an ionic bond.
Polarity/Electronegativity - atoms may or may not share the valence electrons equally. In nonpolar covalent bonds, the valence electron is shared evenly between the two atoms. In polar covalent bonds, one atom has greater electronegativity, or ability to attract an electron more strongly than the other atom. This results in one atom having a partial negative charge while the other atom has a partial positive charge.¹ The polarity of atoms is important in determining if they are water-soluble. Water is considered polar and can interact with many atoms/molecules.
Hydrogen Bonds - the partial positive hydrogen attracts a partial negative charge of another atom, typically oxygen or nitrogen, causing the formation of a bond.¹ This is weaker than both ionic and covalent bonds but necessary for many compounds, including carbohydrates, lipids, and protein.
How do atoms and molecules cause reactions within the body?
Several chemical reactions occur within the body to sustain life. Bonds between atoms must be formed or broken.
Bonds represent potential energy or stored energy. Bonds must break to release energy and are often exergonic (releasing more energy than absorbed) and catabolic (breaking down substances into simpler components).¹
An example of a catabolic, exergonic chemical reaction is a decomposition reaction. This can be summarized as the following:
AB -> A + B
When bonds are formed, the chemical reaction is often endogenic (absorbing more energy than released) and anabolic (simpler components building complex structures).¹ An example of an anabolic, endergonic reaction is a synthesis reaction. This can be summarized as the following:
A + B -> AB
Oftentimes, reactions are both catabolic and anabolic. An example of this is an exchange reaction which can be generalized as:
AB + CD -> AD + BC
AB and CD are broken (catabolic) and AD and BC are synthesized (anabolic).
A reversible reaction is a reaction that can occur in either direction and be catabolic and anabolic as well. The following depicts a reversible reaction:
AB <-> A + B
The double-headed arrow indicates AB is broken down into A and B products, and A and B can be combined to form AB.
Oxidation-reduction reactions are plentiful within the body. When oxidizing, the atom loses electrons and releases energy (exogenic, catabolic).¹ When reduced, the atom gains electrons and absorbs energy (endogenic, anabolic).¹These are considered ionic bonds.
How fast or slow do chemical reactions occur within the body?
Atoms and molecules are constantly moving and colliding. Reactions occur when the collision is significant enough to disrupt the valence electrons, breaking or forming bonds which signifies a chemical reaction. A term known as ‘activation energy’ is the amount of energy required to break bonds.¹
Chances of a chemical reaction occurring are influenced by body temperature (high body temperature leads to more collisions) and concentration (more atoms/molecules in an area cause more collisions).¹
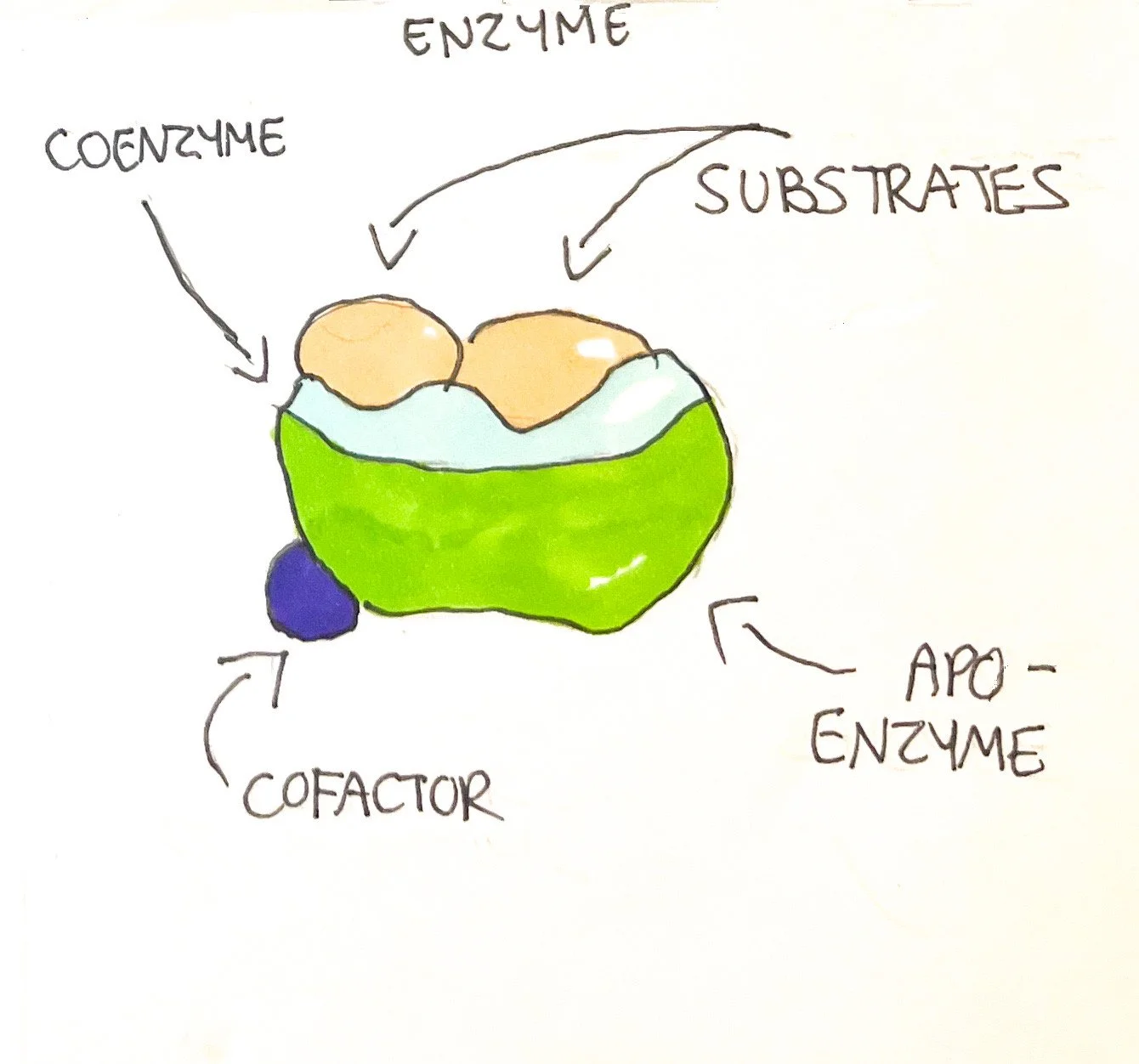
However, the collisions for some atoms/molecules must occur at specific points. Also, chemical reactions must happen at a substantial rate to maintain life. Catalysts, such as enzymes, play a significant role in orienting atoms/molecules by binding specific substrates (reactants) to their specialized active site. This in turn allows for the enzyme to catalyze substrates to products amounting anywhere between 1-600,000 in roughly a second.¹
Organic Molecules: Understanding and Recognizing Molecular and Structural Formulas of Organic Atoms/Molecules
Molecular/Condensed Formulas
The molecular formula of a molecule tells the type of atom and amount. The subscript identifies the amount of an atom. An atom without a subscript indicates one. The superscript identifies charge in the case of ions. Molecules have no overall charge because the combination of atoms cancel out a positive or negative charge; however, some atoms within the molecules may exhibit polarity or electronegativity.
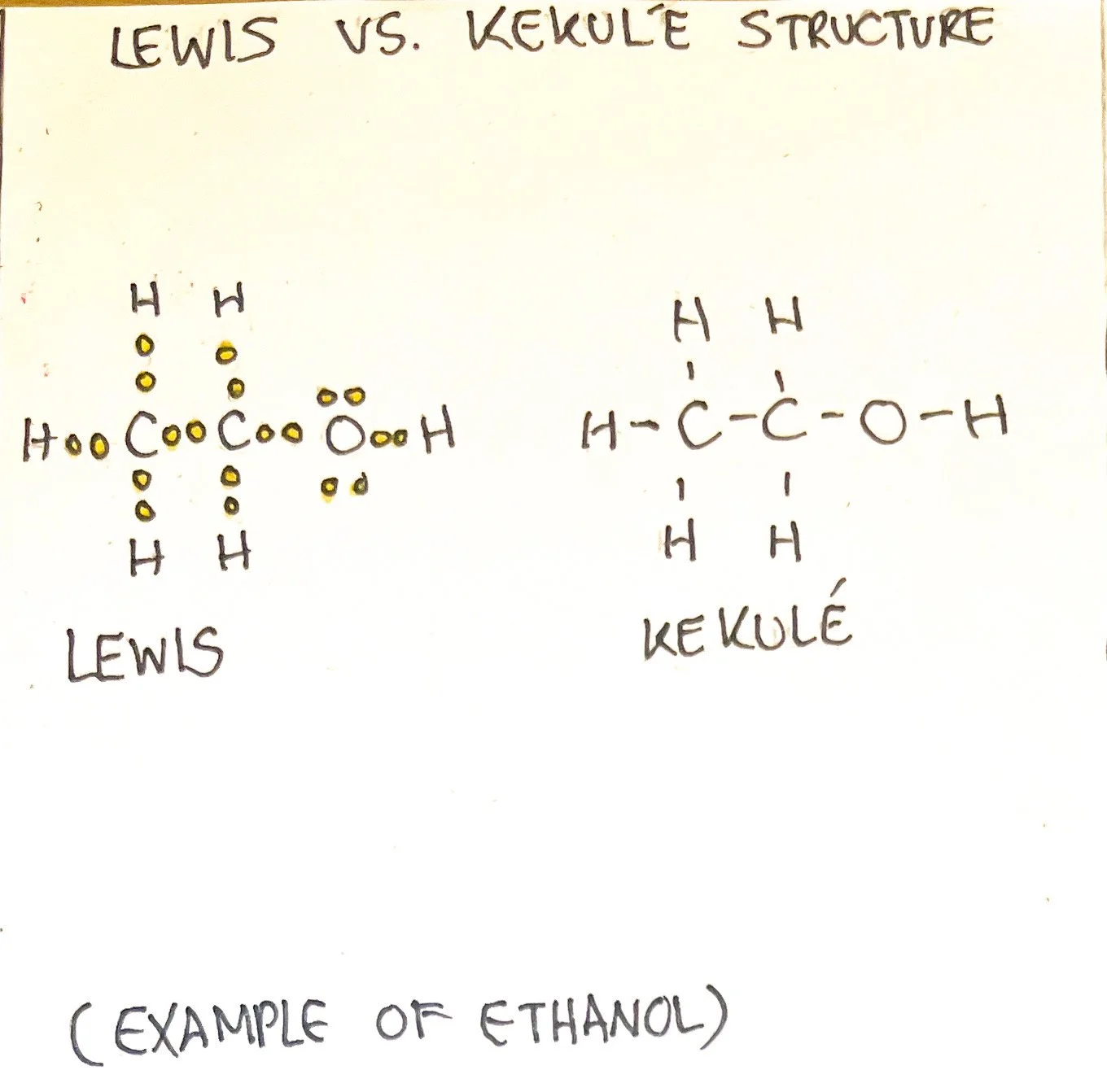
An example of the molecular formula of methanol is CH₄O. A condensed formula shows the order of bonding between molecules. For example, methanol written out in a condensed form would be CH₃OH. Different structures with the same formula are called isomers.² This is when the molecular formulas of molecules are the same, but the types of molecules are different. This is due to the structural arrangement of the molecules.
Structural Formulas
Atoms and molecules can be drawn using Kekulé or Lewis structures. Lewis structures use dots to represent electrons and depict the unbonded electrons. The Kekulé structure uses lines to represent electron pairs and does not depict unbonded electrons.³ However, the Lewis structure may also contain lines while the Kekulé structure may contain dots as they are often drawn in different manners. Bond-line formulas are used to show carbon bonds among other functional groups. ³ The condensed formula makes it easier to draw these structures considering that it depicts how atoms are arranged.
Five Rules to Understanding Structural Formulas of Molecules
There are rules to consider when interpreting structural formulas of diatomic molecules (two atoms bonded), polyatomic molecules, (three or more atoms covalently bonded), and ions (molecules with a [+] or [-] charge).
1. The amount of bonding that each atom can have is related to its valence electrons. Except for hydrogen, molecules are striving to achieve the octet rule whether donated or shared. The amount of valence electrons for some atoms is listed below:
Carbon: 4 valence electrons
Nitrogen: 5 valence electrons
Oxygen: 6 valence electrons
Hydrogen: 1 valence electron
2. The line between two atoms represents two valence electrons. If atoms share two valence electrons, it has one line representing a single bond. If atoms share 4 valence electrons, it has two lines representing a double bond. If atoms share 6 valence electrons, it has three lines representing a triple bond.
3. If an atom has atoms/molecules branching out from it, it is considered the central atom. It should be given precedent and have eight electrons around it.³ Carbon atoms are central while hydrogen atoms are terminal, or located at the end.³
4. Ideally, atoms are looking to satisfy the octet rule (except for hydrogen which is satisfied with two electrons). Some electron pairs may not be bonded. Single electrons in an atom or molecule may not be bonded and are highly reactive - this is known as a free radical.³ The Lewis structure shows unbonded electrons.
5. A polyatomic ion charge can be determined by looking at the superscript of a molecule. A negative charge is located among the most electronegative atoms (attracts and therefore gains an electron) while a positive charge is located among the least electronegative ion (less attraction and therefore losing an electron).³
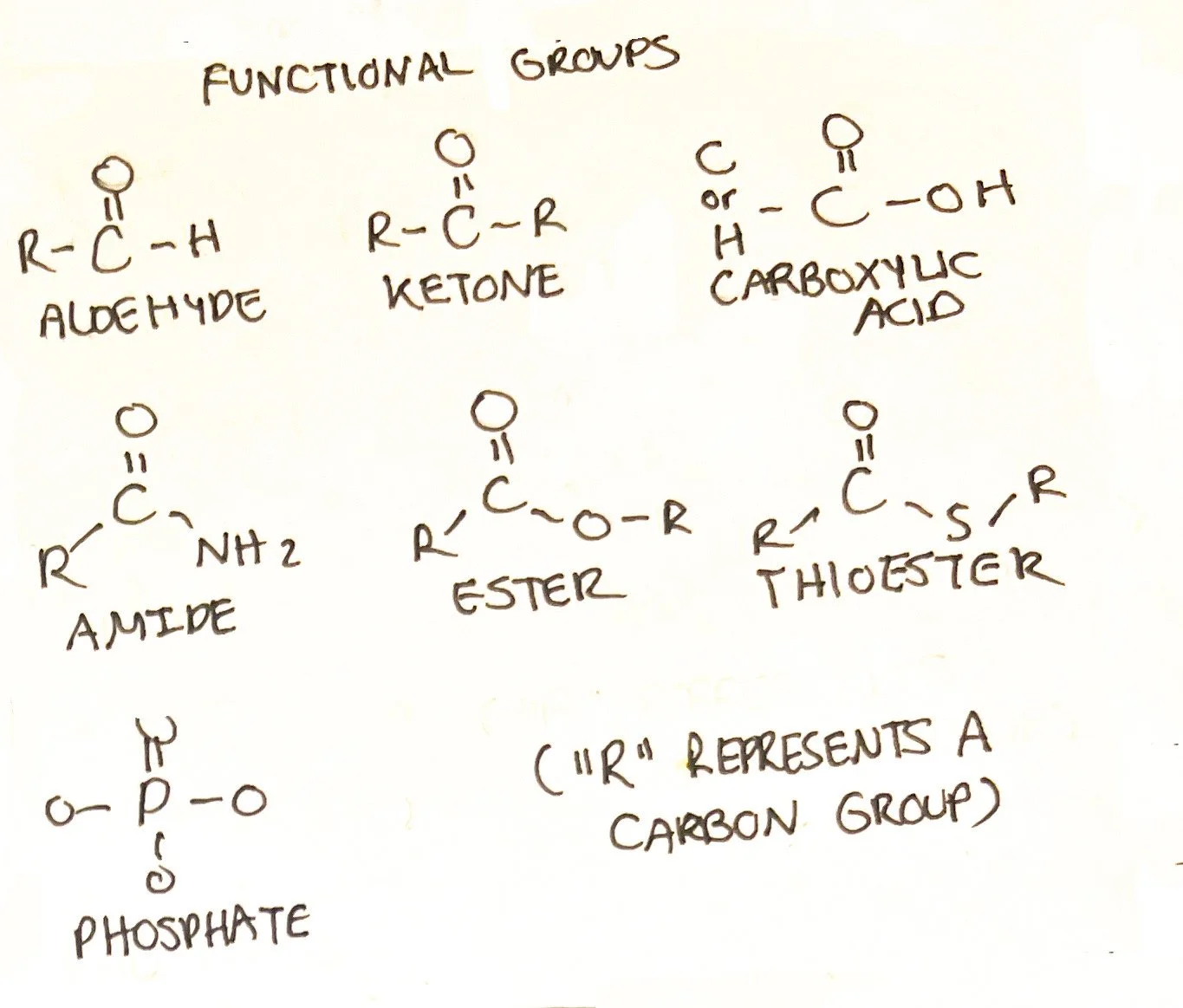
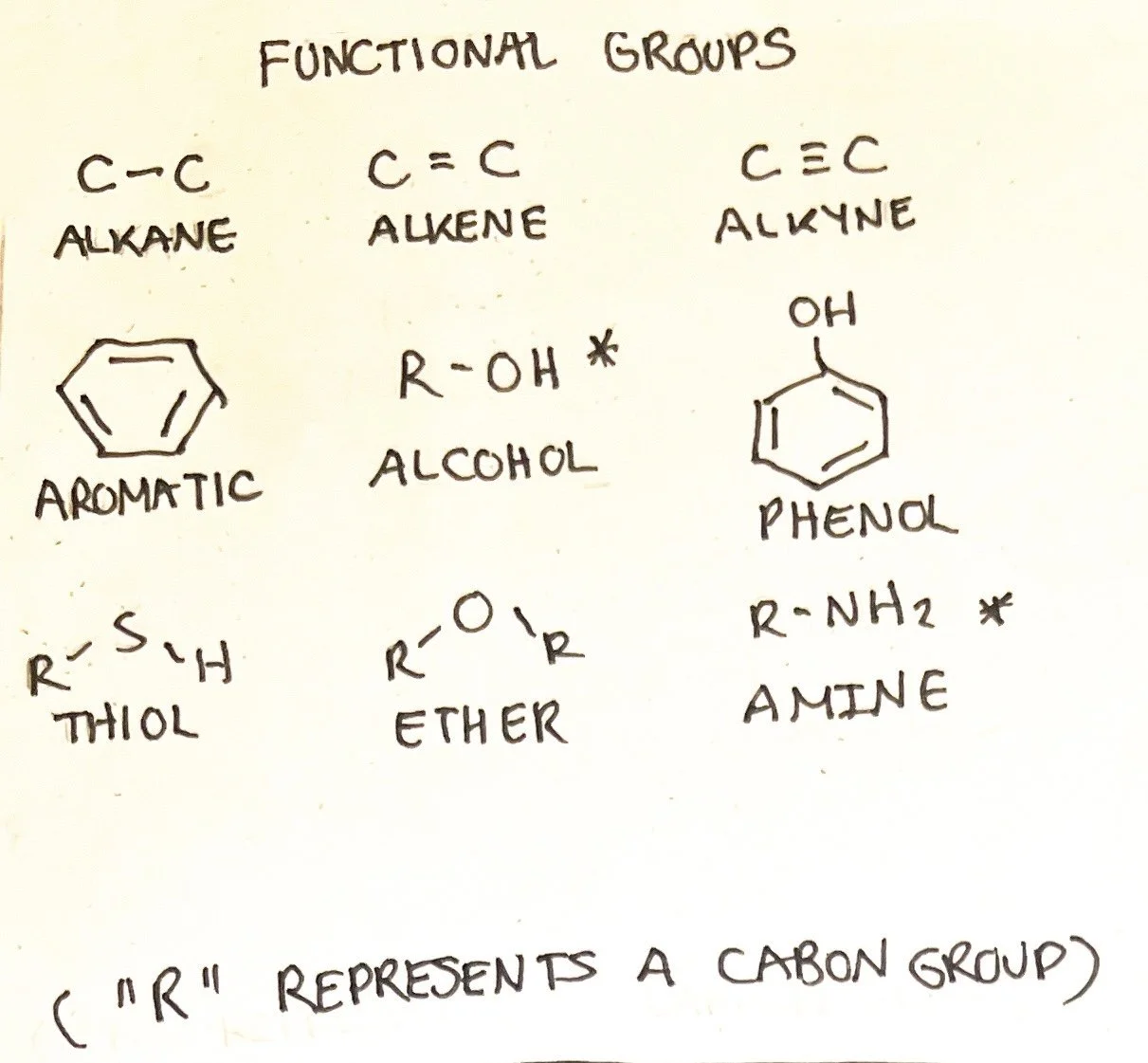
Functional Groups
Some molecule arrangements can be associated with specific functions that can predict how they will interact within the molecule or with other molecules. These are known as functional groups. Many molecules contain more than one functional group within its structure. Below are some functional groups that are commonly seen within the molecules in our bodies:
Alkanes, Alkenes, and Alkynes
Alkanes (alkyl), alkenes, and alkynes form the chainlike structures found within many nutrients, including carbohydrates, proteins, and lipids. Despite being listed as a functional group, alkanes are not typically associated with specific functions of their own and are not always considered a functional group. Alkanes (alkyl), alkenes, and alkynes are considered nonpolar; however, alkenes and alkynes are weakly polar at times.
Alkanes/Alkyls - Alkanes are hydrocarbon molecules that contain a single bond. Hydrocarbons are carbon atoms attached to hydrogen atoms. Alkanes can also be in ring-like structures called cycloalkanes.⁴ Alkanes end in the suffix -ane.⁴ Alkanes are considered saturated hydrocarbons and are found within saturated fatty acids. Alkyls are alkanes with a hydrogen group missing. The suffix of alkyls is -yl.
Alkenes - Alkenes are hydrocarbon molecules with a double bond. The suffix -ene indicates an alkene.⁴ Alkenes are considered unsaturated hydrocarbons due to the double bond limiting the bonding of hydrocarbon molecules. These are found within unsaturated fatty acids.
Alkynes - Alkynes are hydrocarbon molecules with triple bonds. The suffix -yne indicates an alkyne.⁴ Alkynes are also considered unsaturated hydrocarbons. These are found within unsaturated fatty acids.
Aromatics - Aromatics form a carbon ring structure that can have alternating double bonds with different arrangements. Generally, they are considered nonpolar.⁵
Alcohols - Alcohols are identified by hydroxyl (-OH) bonded to carbon. R represents any other molecule bonded (typically carbon and hydrogen groups) and differentiates molecules. Alcohols can be classified based on whether the carbon atom bonded to -OH contains one additional carbon group (primary), two carbon groups (secondary), or three carbon groups( tertiary). Alcohols have the suffix -ol. This functional group is found within carbohydrates and lipids. Alcohols are polar molecules.⁵
Phenols - Phenols are when an aromatic ring is directly bonded to an alcohol group (-OH). Phenols are polar.⁵
Thiols - Thiols contain a carbon attached to a sulfur molecule and can be classified as primary, secondary, or tertiary similar to alcohols. Thiols are more nonpolar than polar.⁵
Ethers - In ethers, an oxygen molecule is central and bonded to two carbon groups on either side. Ethers are slightly polar.⁵
Amines - The central atom is nitrogen with carbon groups. Similar to alcohol, it is classified as primary, secondary, and tertiary. However, the classification is based on how many carbon groups are bonded to the nitrogen atom.⁵ Amines often form ammonium ions that may or may not have carbon groups. Protein structures, such as amino acids, contain amines. Amines are polar molecules.⁵
Aldehyde - An aldehyde contains a carbonyl group (carbon double bonded to oxygen) which is bonded to one other carbon group. Aldehydes can be found within the structure of carbohydrates. Aldehydes are polar molecules.⁵
Ketone - A ketone contains a carbonyl group which is bonded to two carbon groups. Ketones can be found within the structure of carbohydrates and some lipids. Ketones are polar molecules.⁵
Carboxylic acid - A carbonyl group bonded to an -OH group and either a carbon or hydrogen. Carboxylic acid is interchangeable with carboxylate ions which are found in amino acids. They are highly polar. ⁵
Amides - A carbonyl group bonded to a nitrogen atom. Found within some lipid structures but are essential for protein. Amides are polar molecules.⁵
Esters - A carbonyl group bonded to another oxygen atom. Found within many lipid structures.⁵
Thioesters - A carbonyl group bonded to a sulfur atom. Esters are polar molecules.⁵ ⁶
Phosphates - Phosphates contain a central phosphate atom and can be diphosphates or triphosphate groups. Phosphates are generally polar.⁵ ⁶
Sources:
1. Jerry Tortora and Bryan Dickerson, Principles of Anatomy & Physiology, 16th ed. (New Jersey: John Wiley & Sons, 2021).
2.https://chem.libretexts.org/Courses/Saint_Francis_University/CHEM_113%3A_Human_Chemistry_I_(Muino)/04%3A_Molecular_Compounds/4.06%3A_Molecular_Formulas_and_Lewis_Structures
5.https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Chemistry_for_Allied_Health_(Soult)/04%3A_Structure_and_Function/4.04%3A_Functional_Groups
6. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/03%3A_Organic_Compounds-_Alkanes_and_Their_Stereochemistry/3.01%3A_Functional_Groups