Lipids (Fats & Oils)
You should be able to:
Identify the different categories of lipids and their chemical compositions.
Learn the recommendations for lipids and how they can be tailored based on energy intake.
Understand where lipids are primarily found in foods.
What are fat and oils?
Fat and oil, part of a group known as lipids, are composed of carbon, hydrogen, and oxygen molecules. These form several types of lipids with different functions, such as fatty acids, triglycerides, phospholipids, and steroids.¹ At room temperature, fat is solid and oil is liquid; this is related to the saturation of the fatty acids. Other than steroids, most structures are composed of a glycerol backbone with fatty acids. There are two essential fatty acids necessary for humans - linoleic acid and alpha-linolenic acid - which are considered the foundation for omega-6 and omega-3 fatty acids.¹
What are the types of lipids?
There are several types of lipids with most compounds following the same structure. Below are some common types of lipids:
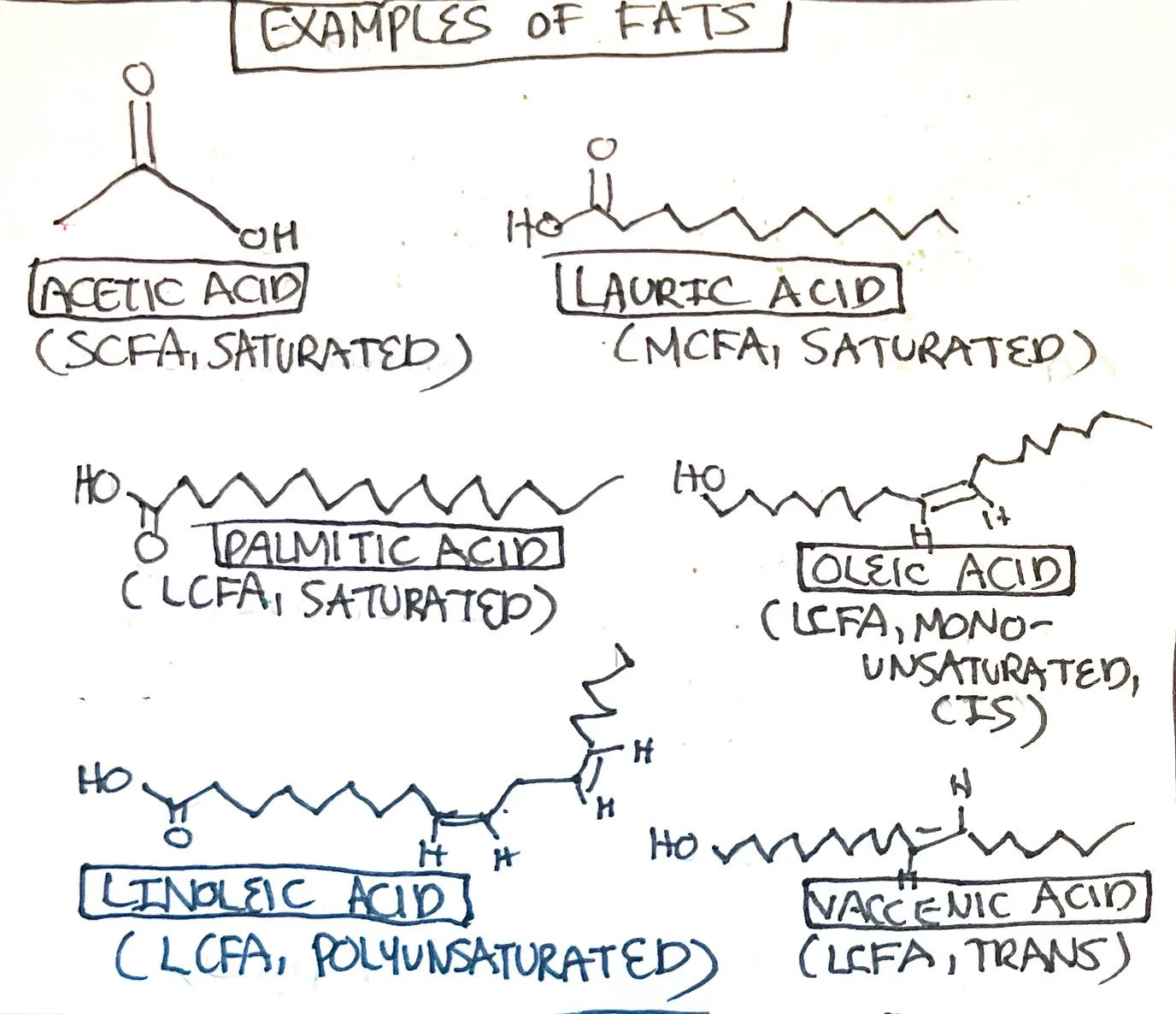
Fatty Acids
Fatty acids are composed of a hydrocarbon chain. The amount of carbons within a fatty acid can range between 8-22+.² The fatty acid has a polar end, which is a carbonyl group (C=O), and a nonpolar end which is a methyl end (-CH3). The suffix of fatty acids ends in -ate or -ic. Fatty acids can be categorized by the amount of carbons:
Short-chain fatty acids (SCFA): 2-4 carbons
Examples include acetate, propionate, and butyrate.³
Medium chain fatty acids (MCFA): 6-12 carbons
Examples include caproic acid, caprylic acid, and lauric acid.³
Long-chain fatty acids (LCFA): 14-26 carbons
Examples include palmitic acid, oleic acid, linoleic acid, and alpha-linolenic acid.³
Fatty acids can also be classified based on degree of saturation, including:
Saturated Fatty Acids: fatty acids that have no double bonds. The carbon is ‘saturated’ with hydrogen atoms.
Due to saturation, these fatty acids are more likely to be solid at room temperature. They also have a higher melting point.
Examples include acetic, butyric, caproic, caprylic, lauric, myristic, palmitic, stearic, and arachidic.³
Unsaturated Fatty Acids: fatty acids with one or more double bonds. The double bond allows for less hydrogen bonding to carbon.
Due to less saturation, these fatty acids are more likely to be liquid at room temperature and more soluble in water.
Monounsaturated Fatty Acids: fatty acids with one double bond
Examples include palmitoleic and oleic.³
Polyunsaturated Fatty Acids: fatty acids with more than one double bond
Examples include linoleic, alpha-linolenic, arachidonic, eicosatetraenoic, docosahexaenoic.³
The orientation of the hydrogen on the double bond further categorizes fatty acids. For example, if the hydrogen bond is aligned along the double bond, it is considered to be in a cis position. If the hydrogen bond is on the opposite side of the double bond, it is considered to be in a 'trans' position. The cis fatty acids have a kink, and trans fatty acids are straighter. In food manufacturing, unsaturated fats (oils) are hydrogenated resulting in the production of trans fat which extends shelf life and turns it solid.² Trans fats are also found in dairy and meat products naturally. Trans fats are known to have health consequences, such as raising LDL cholesterol and negatively impacting lipid enzyme functionality, fatty acid chain elongation, and quantity of fatty acids.³
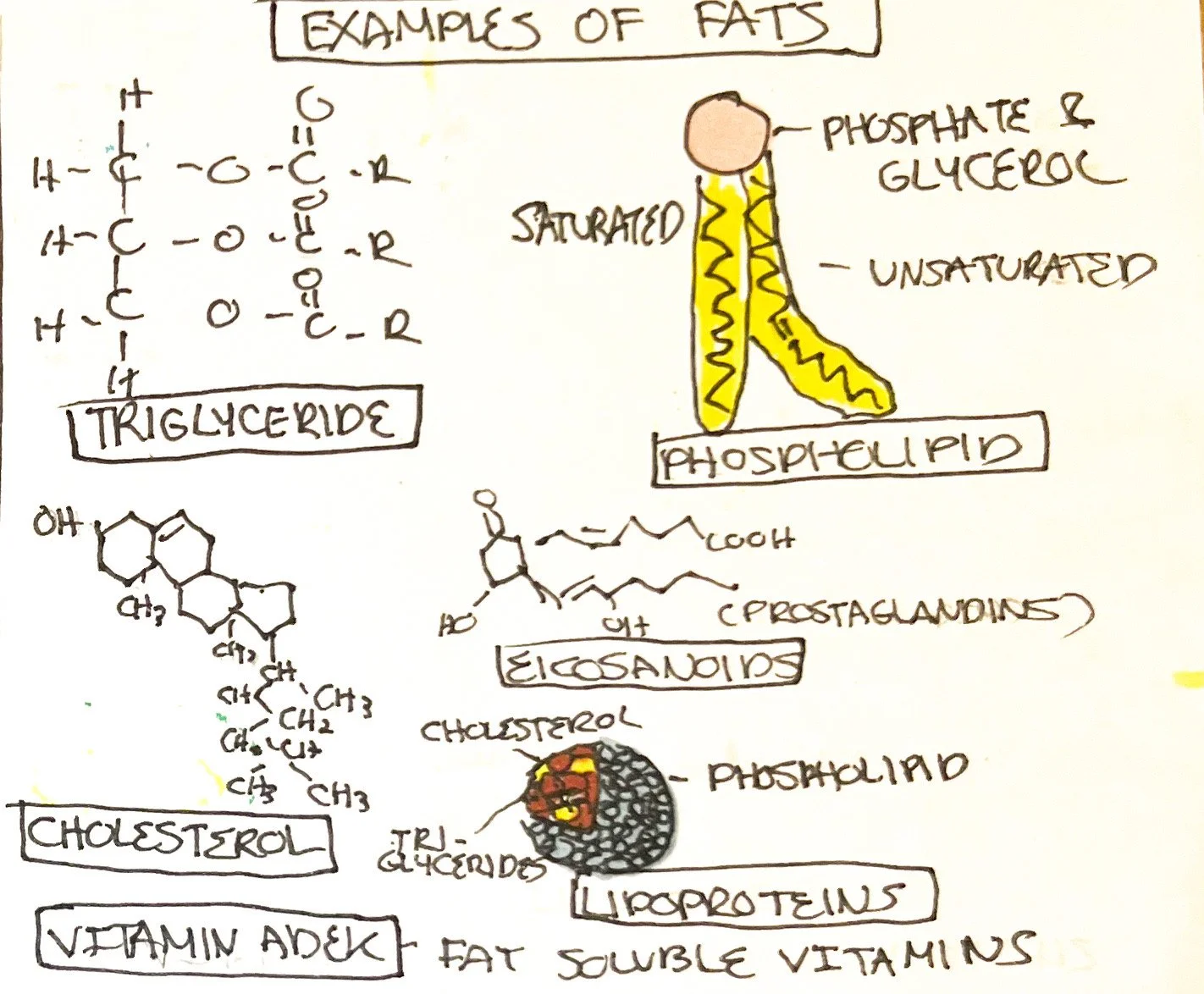
Triglycerides
A glycerol backbone is bonded to three fatty acids via an ester bond in triglycerides. The glycerol backbone is composed of three carbons. Triglycerides are formed via dehydration synthesis reactions and can be separated with hydrolysis reactions.¹ Triglycerides are found in food and in the body.
Phospholipids
Similar to a triglyceride, a phospholipid has fatty acids and a glycerol backbone. Replacing the third fatty acid is a phosphate group (PO43-) which typically bonds a small group containing nitrogen.¹ The phosphate group is considered the polar head and is hydrophilic, or attracts water. The fatty acid ‘tails’ are nonpolar and hydrophobic (repels water). The term for a compound that has both fat and water-soluble properties is known as amphiphilic.³
Cholesterol/Sterols
Cholesterol consists of four carbon rings, a hydrocarbon tail, and a hydroxyl group (called sterols). The hydroxyl group makes the sterol slightly amphiphilic.¹ All steroid hormones are derived from cholesterol, including estrogen, testosterone, cortisol, etc. Cholesterol is required for cell membrane structure and bile formation.¹
Eicosanoids
Eicosanoids are lipids derived from arachidonic acid, consisting of prostaglandins and leukotrienes responsible for a variety of functions, including inflammatory and hormonal responses.¹
Fat Soluble Vitamins and Lipoproteins
Vitamins A, D, E, and K are considered lipids. Lipoproteins are spherical complexes containing fatty acids, cholesterol, and triglycerides inside surrounded by phospholipids and proteins functioning as transportation for cholesterol among other functions.¹
What is the function of lipids in the body?
Lipids serve a variety of functions within the body. Fatty acids synthesize other lipid structures such as triglycerides and phospholipids, but they can also be metabolized for ATP.¹ Alpha-linolenic acid, an essential fatty acid, derivatives include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).¹ EPA is found within phospholipids, cholesterols, and triglycerides while DHA is found within phospholipids, brain, eyes, and testes.³ The other essential fatty acid, linoleic acid, is converted to arachidonic acid.¹ This fatty acid is found largely within the central nervous system.¹ Linoleic acid can be further converted to intermediate products to produce EPA and DHA.³ However, conversion rates from both essential fatty acids to DHA and EPA are low.³ Triglycerides stored as body fat can be used as a source of energy when carbohydrate stores (glycogen) are low. Fat within the body also insulates and cushions vital organs. Phospholipids compose cell membranes with a unique amphiphilic structure. Eicosanoids play several roles within the body from muscle contractions to inflammatory responses.¹ Cholesterol is also part of the cell membrane but is the foundation for all steroid hormones and necessary for bile production.¹ Other lipids, such as some vitamins and lipoproteins, range in functions such as supporting calcium regulation, blood clotting, and transporting.¹
What is the recommended amount of lipids?
The AMDR is reflective of dietary patterns of nutrients while the RDA focus is on meeting the requirement for daily functioning. The AMDR recommended ranges for lipids as a percentage of total calories are as follows⁴:
Children:
1-3 years old: 30-40%
Children/Teens:
4-18 years old: 25-35%
Adults:
18+ years old: 20-35%
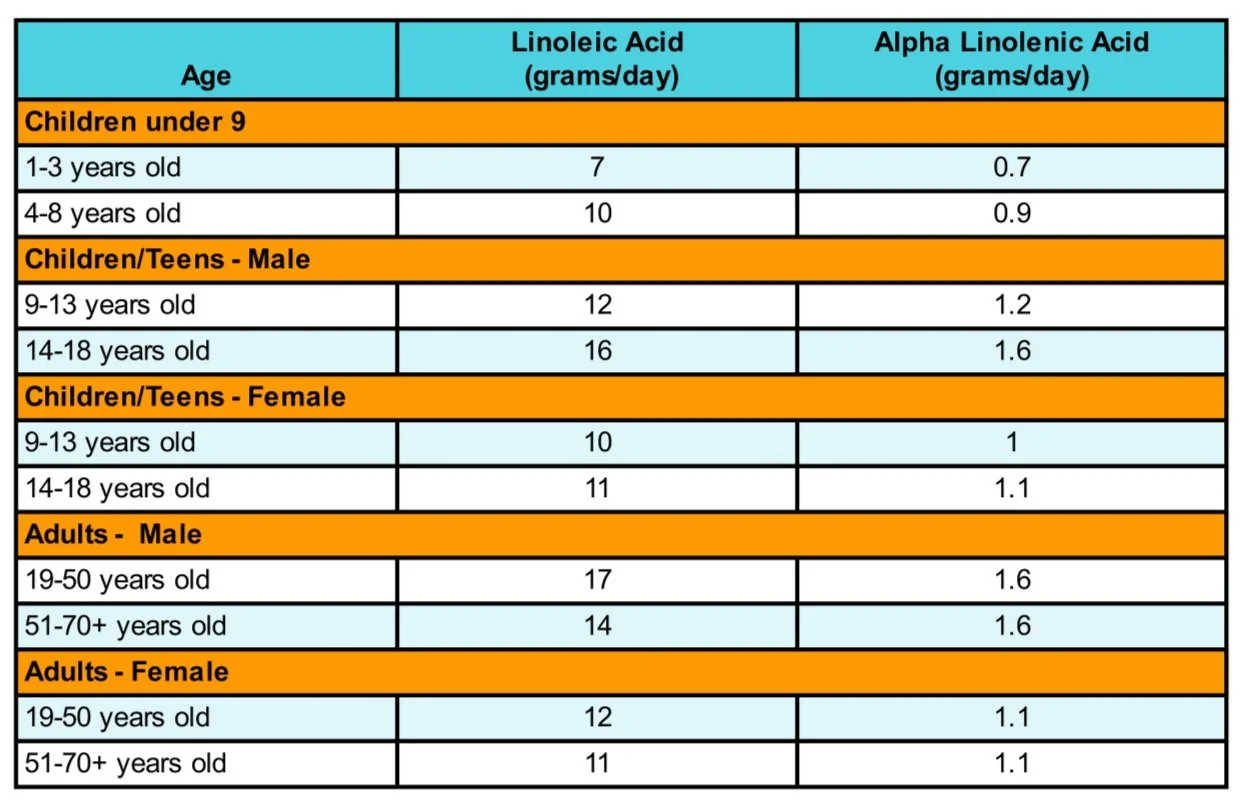
The RDA amount for fat is not determined for those 1 and older. However, there is an AI for linoleic and alpha-linolenic acid per day. AI is similar to RDAs but has less scientific information to define it as such.³ The following are recommendations³:
Lipids contain 9 calories per gram.
Determining Kcals, Grams, and Servings of Lipids When Daily Calorie Requirement is Known.
To determine the amount of calories from fat, multiply your daily calorie requirement by the AMDR.
This provides the amount of calories from lipids.
Example: 2000 kcals/day
1. 2000 kcals/d x 20% = 400 kcals from lipids
In order to determine grams of lipids, divide kcals from lipids by 9 (9 kcals per gram)
2. 900 kcals from lipids/ 9 = 44 grams of fat.
What foods contain lipids?
Foods that are known to contain lipids include meats/fish, cheese, milk, spreads (butter, margarine), dressings/oils, and nuts. Grain, fruits, and vegetable products are not a significant source of lipids. Linoleic acids are found in meats and vegetable oils; alpha-linolenic acids are found in certain types of fish (cold, fatty), seeds (flaxseed), and oils (canola, fish).¹ ²
Source(s):
1. Jerry Tortora and Bryan Dickerson, Principles of Anatomy & Physiology, 16th ed. (New Jersey: John Wiley & Sons, 2021).
2. Judith E Brown. Nutrition Through the Life Cycle, 6th ed. (Boston: Cenage Learning, 2017), 1-50.
3. Denise M Medeiros and Robert E.C. Wildman, Advanced Human Nutrition, 4th ed. (Burlington, MA: Jones & Bartlett, 2019).
4. https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx#dri