Science of Metabolism
You should be able to:
Identify the three main processes of cellular respiration
Learn about glycogen anabolism and catabolism.
Explore important metabolic molecules that are necessary for metabolism.
Understand lipid and protein metabolism (anabolism and catabolism processes).
After the food you eat is broken down and absorbed as monosaccharides, fatty acids, and amino acids, what happens next? Within the body, each nutrient undergoes a variety of processes to sustain life. Metabolism, which is the collective sum of reactions that occur in the body, can either be catabolic (the breakdown of products releasing energy) or anabolic (building products that require energy).¹ The energy molecule, adenotriphosphate (ATP), is formed during catabolic reactions when energy is released. Some energy is released as heat.¹ When molecules combine in anabolic reactions, ATP is used to create complex structures and also releases heat.¹ This guide will describe the processes of glucose, fatty acids, and amino acids and what roles they play within the body.
Carbohydrate Metabolism
After absorption, monosaccharides such as galactose and fructose are converted within liver cells (hepatocytes) to glucose. Glucose enters cells using GluT transporters via facilitated diffusion.¹GluT transporters are found within most body cells; specifically, the GluT4 transporter is inserted within body cells as insulin (transporting sugar from the blood into cells) levels increase.¹ Carbohydrate metabolism is the metabolism of glucose.
Glucose Metabolism
Cellular Respiration: Glucose Catabolism
Glucose is catabolized to create ATP in a series of processes called cellular respiration. The three processes that glucose can undergo are known as glycolysis, Krebs cycle reactions, and electron transport chain reactions.
Glycolysis
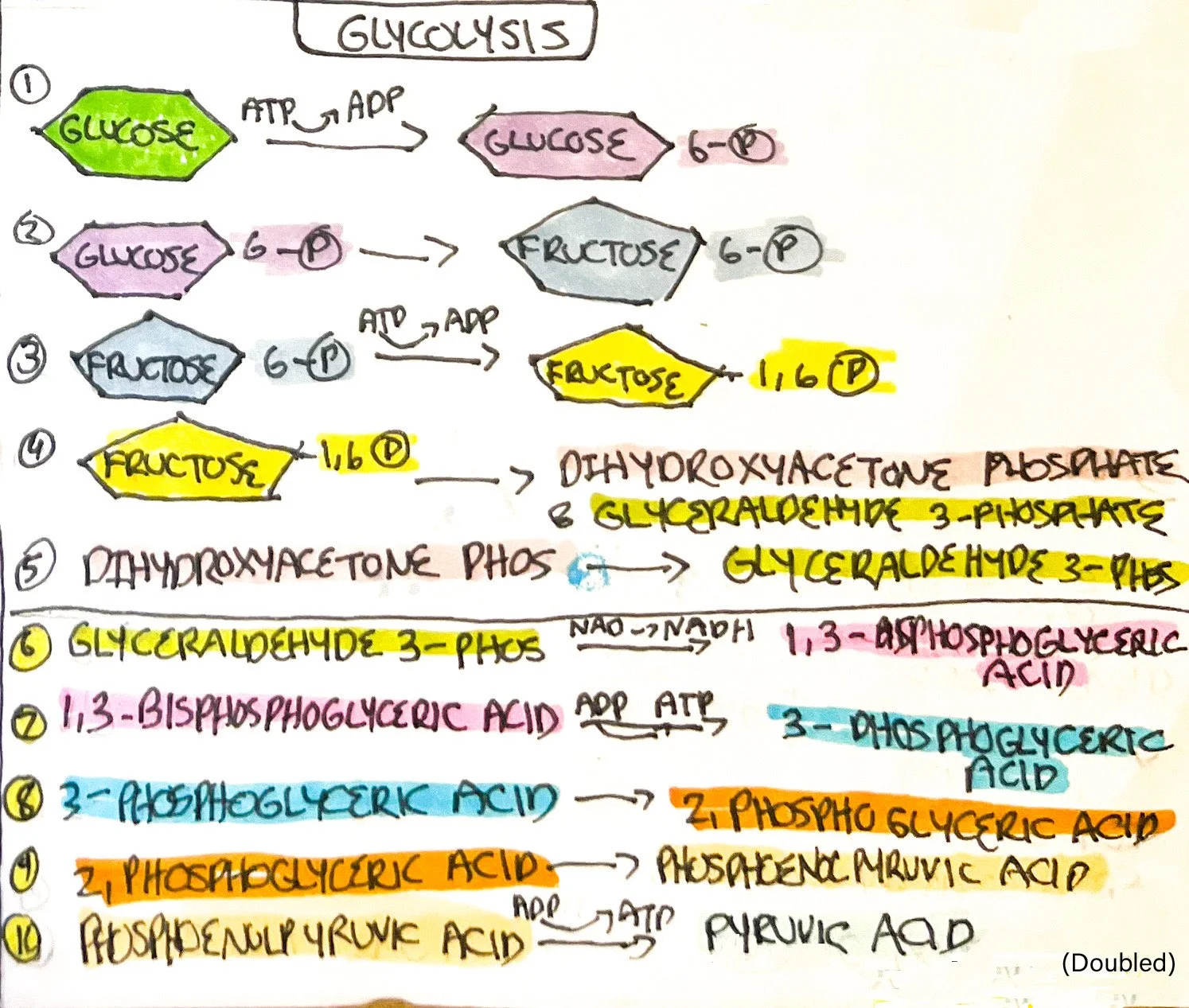
Glycolysis is the breakdown or catabolizing of glucose into pyruvic acid used within the Krebs cycle and electron transport chain. There are ten reactions that make up glycolysis, occurring in the cytosol of the cell. Glycolysis can occur with oxygen (aerobic) or without oxygen (anaerobic).¹ If there is a lack of oxygen available within the body, such as when exercising, the final product - pyruvic acid - is converted into lactic acid (reaction is reversible). The buildup of lactic acid is known to cause muscle cramps during exercise.
Key principles found within glycolysis include oxidation-reduction reactions and ADP conversion into ATP. Oxidation-reduction reactions commonly occur within the body. Oxidation is the removal of electrons which decreases potential energy.¹ In bodily reactions, hydrogen atoms are removed and are known as dehydrogenation reactions.¹ Reduction is the gain of electrons and hydrogen atoms within the body. Nicotinamide adenine dinucleotide (NAD) derived from B vitamin niacin, and flavin adenine dinucleotide (FAD) derived from B vitamin riboflavin are reduced into NADH+H⁺ and FADH2 to be used within the electron transport chain.¹
The steps are listed below:
Glucose is phosphorylated (adding a phosphate group) to glucose 6-phosphate*, consuming 1 ATP (ATP->ADP).¹
Glucose 6-phosphate is converted to fructose 6-phosphate.¹
Fructose 6-phosphate is phosphorylated to fructose 1,6-bisphosphate, consuming 1 ATP (ATP->ADP). An important enzyme, known as phosphofructokinase, catalyzes this reaction.¹
Fructose 1,6-bisphosphate splits into dihydroxyacetone phosphate and glyceraldehyde 3-phosphate (G 3-P).¹
Dihydroxyacetone phosphate converts into G 3-P, forming 2 molecules of G 3-P.¹
Each G 3-P is converted into molecules of 1,3-Bisphosphoglyceric acid. Two NAD+ are reduced to NADH and hydrogen atoms.¹
Each 1,3-Bisphosphoglyceric acid is converted into 3-Phosphoglyceric acid, forming 1 molecule of ATP per molecule (ADP->ATP).¹
Each 3-phosphoglyceric acid is converted into 2-phosphoglyceric acid.¹
Each 2-phosphoglyceric acid is converted into phosphoenolpyruvic acid.¹
Lastly, each phosphoenolpyruvic acid is converted into pyruvic acid, forming two molecules of the end product of glycolysis. 1 molecule of ATP is formed per molecule (ADP->ATP).¹
The end results of glycolysis are as follows:
ATP consumed (ATP->ADP): 2
ATP formed (ADP->ATP): 4
Redox reactions (NAD->NADH+ H⁺): 2
Pyruvic acid formed: 2
Formation of Acetyl Coenzyme A
If oxygen is not available, the pyruvic acid will be reduced using the anaerobic pathway. The NADH formed in step 6 of glycolysis will be oxidized into NAD when oxidizing pyruvic acid into lactic acid.¹ The regeneration of NAD allows glycolysis to continue. In states of low oxygen such as when exercising, more lactic acid forms and enters the blood. Hepatocytes (found in the liver) remove lactic acid from the blood and convert it into pyruvic acid to prevent the buildup of lactic acid in the blood.¹ The buildup of lactic acid induces muscle fatigue when exercising.
If oxygen is available, the pyruvic acid will be prepped to enter the Krebs cycle. Occurring in the mitochondria, pyruvic acid undergoes decarboxylation, or the removal of carbon dioxide (CO2).¹ The resulting acetyl group is attached to a derivative of pantothenic acid, or vitamin B5, forming the beginning product of the Krebs cycle: acetyl coenzyme A (acetyl CoA).¹ During this process, NADH and hydrogen are formed and carbon dioxide is released. Since there are two pyruvic acid molecules, 2 NADH and hydrogen molecules are formed.
Krebs Cycle
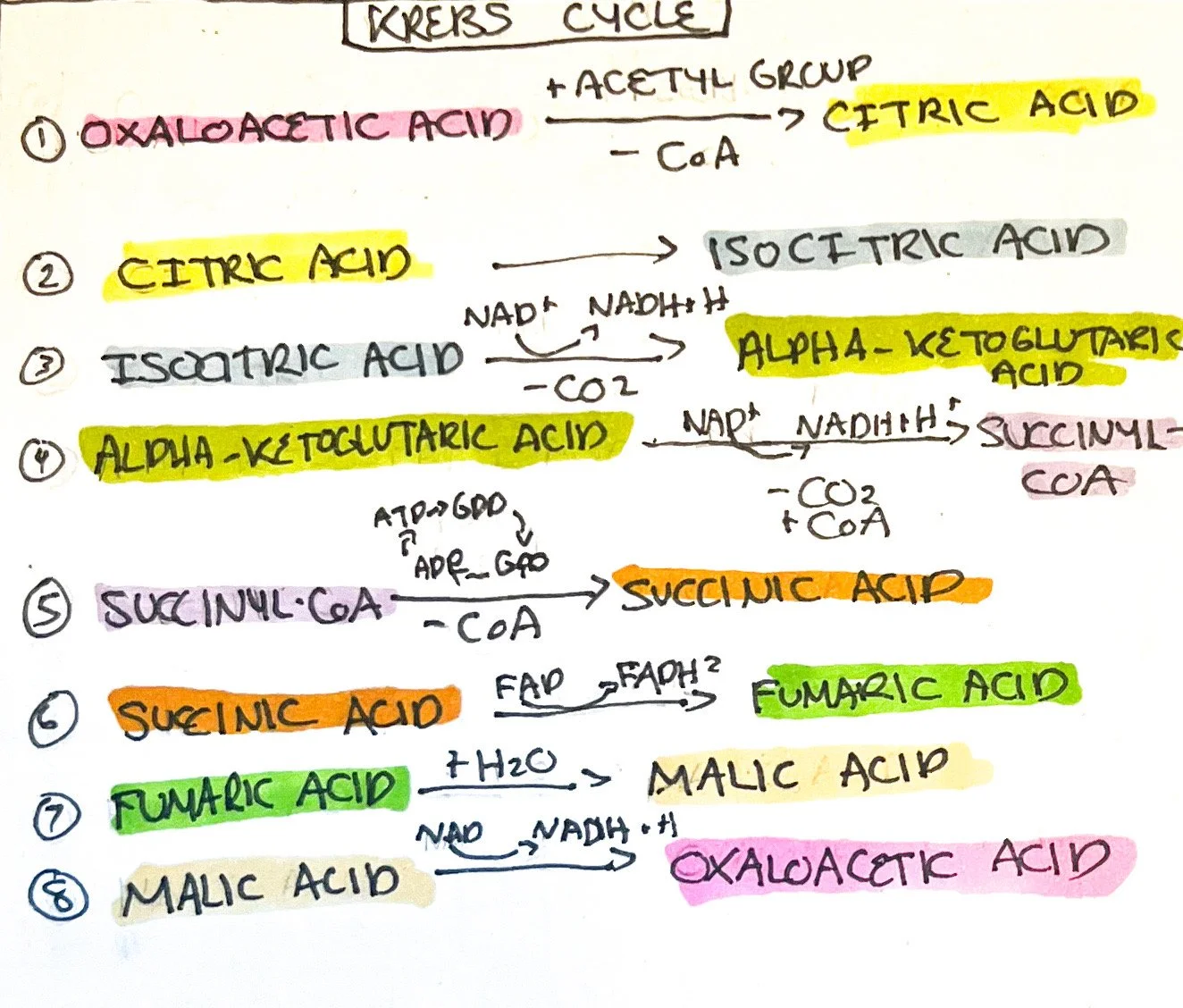
The Krebs cycle occurs within the mitochondrial matrix and is a series of redox reactions (harnessing hydrogen ions for the electron transport chain) and decarboxylation reactions (removal of carboxyl group releasing carbon dioxide). Eight steps occur during the process, described below:
The acetyl group of acetyl CoA enters the Krebs cycle and combines with four-carbon oxaloacetic acid and water to form citric acid. The CoA portion of the acetyl CoA is used to combine with another acetyl group to continue the cycle.¹
Citric acid is converted into isocitric acid by isomerization (same molecular formulas with different structures).¹
Isocitric acid is oxidized into alpha-ketoglutaric acid by decarboxylation, releasing carbon dioxide and producing NADH and hydrogen.¹
Alpha-ketoglutaric acid is oxidized into succinyl-coA with the addition of Coenzyme A and the release of carbon dioxide. NADH and hydrogen are formed.¹
Succinyl-CoA is converted into Succinic acid by the removal of Coenzyme A and ATP is formed (ADP -> ATP) from the conversion of guanosine diphosphate to guanosine triphosphate (GDP -> GTP).¹
Succinic acid is oxidized into fumaric acid and FADH2 is formed.¹
Fumaric acid is hydrated (water molecule added) into malic acid.¹
Malic acid is oxidized into oxaloacetic acid with NADH and hydrogen being formed. Another acetyl CoA combines with oxaloacetic acid to repeat the cycle. Since one glucose molecule produces 2 pyruvate molecules and therefore 2 acetyl CoAs, the cycle repeats twice.¹
The end results of the Krebs Cycle for two glucose molecules are as follows:
CO2 formed: 4
ATP formed (ADP->ATP): 2
Redox reactions (NAD->NADH+ H⁺): 6
(FAD->FADH2): 2
Total Redox Reactions (NAD->NADH+ H⁺): 10
(FAD->FADH2): 2
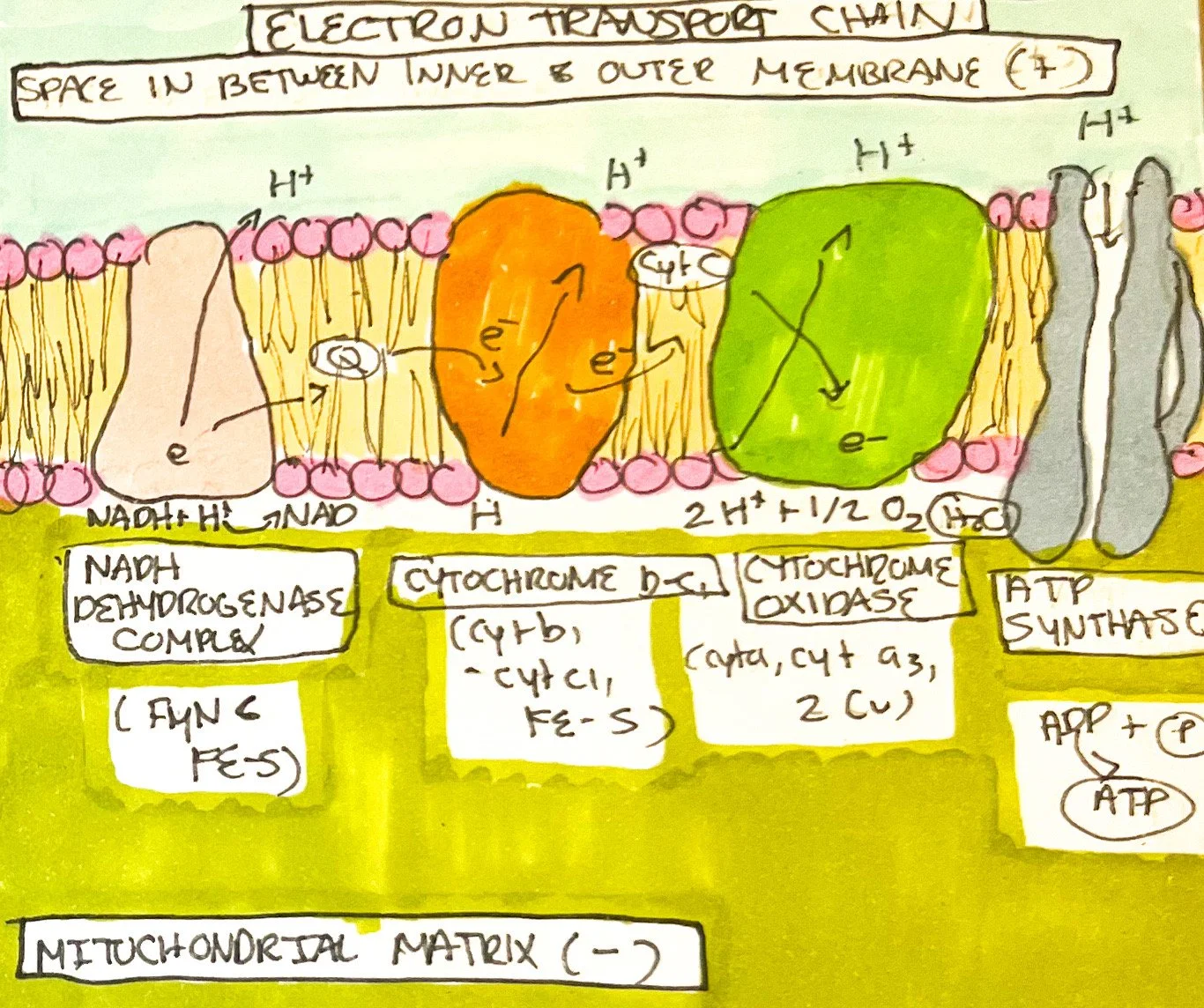
The Electron Transport Chain (ETC)
Multiple sets of integral membrane proteins along the inner mitochondrial membrane form the ETC. Carriers include flavin mononucleotide (FMN), cytochromes, iron-sulfur centers, coenzyme Q, and copper atoms.¹ Electrons move along each carrier as compounds are reduced and oxidized. The carriers form three units that are proton pumps, expelling hydrogen to maintain an electrochemical gradient. The proton pumps are known as NADH dehydrogenase complex (FMN and five iron-sulfur centers); cytochrome b-c1 complex (cyt b1,iron-sulfur center, and cyt c1); and cytochrome oxidase complex (cytochrome c, copper, cytochrome a, and cytochrome a3).¹
As NADH and hydrogen molecules move along each complex and its parts, it is oxidized and hydrogen atoms are channeled through proton pumps.¹ The proton pumps remove hydrogen ions into the area between the inner and outer mitochondrial membranes, forming a difference in electrochemical gradient between the mitochondrial matrix.¹ During this process, the carriers are reduced (such as FMN -> FMNH₂) and oxidized by iron-sulfur centers. Electrons utilize coenzyme Q and cytochrome c to shuttle across each proton pump. The coupling of electron movement and the pumping of hydrogen is known as chemiosmosis.¹
As hydrogen accumulates, special hydrogen channels (including ATP synthase in the inner membrane), allow hydrogen to move back in to form ATP.¹ It does this by using the potential energy stored within the buildup of hydrogen known as proton motive force. Cytochrome a3 passes its electrons to ½ molecule of oxygen and forms a molecule of water after bonding with two hydrogens within the matrix.¹ NADH+H⁺ moving along the ETC produces approximately 2-3 ATPs, and oxidation of FADH2 forms 1-2 ATPs. A total of 26-28 ATPs are formed during the ETC reactions.
Cellular Respiration can be summarized as:
Glucose + 6 oxygen molecules +30-32 ADPS = 6 carbon dioxides, 6 water molecules, and 30-32 ATPs.
Key Metabolic Molecules
There are certain molecules involved in metabolism that must be present in order for other metabolic processes to occur. All molecules mentioned are involved in glucose metabolism but are found in other metabolic pathways that will be described below:
Glucose 6-phosphate: This molecule is first described when glucose is phosphorylated in the first step of glycolysis. Glucose 6-phosphate is responsible not only for glycolysis but gluconeogenesis.¹ Glucose 6-phosphate is also catabolized in cells for glucose release and synthesis of RNA and DNA components.
Pyruvic Acid: This molecule is the end result of glycolysis with two being synthesized per one glucose molecule. Pyruvic acid is reduced into lactic acid (which is converted back into pyruvic acid) during times of muscle stress (ex: exercise).¹ It can be converted into glucose via gluconeogenesis and can create the amino acid alanine.¹
Acetyl Coenzyme A: This molecule is synthesized from pyruvic acid to begin the Krebs cycle. Acetyl Coenzyme A plays a role in lipogenesis and lipolysis.
Glucose Anabolism
Glycogen Anabolism: Glycogenesis
Glucose that is not immediately used for energy production is stored as glycogen - the storage form of glucose - in a process known as glycogenesis. This storage form of glucose is synthesized in the presence of insulin and is found within the liver and muscle cells. The process occurs as follows:
Glucose is phosphorylated into glucose 6-phosphate by the enzyme hexokinase using ATP.
Glucose 6-phosphate is converted into glucose 1-phosphate.
Glucose 1-phosphate is converted into uridine diphosphate glucose.
Uridine diphosphate glucose is converted into glycogen.
Glycogen Catabolism: Glycogenolysis
Glycogen stored within muscle and liver cells is broken down for energy use when signaled by the hormones glucagon and epinephrine. The process is different from the creation of glycogen:
Glycogen is converted into glucose 1-phosphate by phosphorylase which is activated by hormones.
Glucose 1-phosphate is converted into glucose 6-phosphate.
Glucose 6-phosphate is converted into glucose by the enzyme phosphatase and is released into the bloodstream. Phosphorylated glucose is unable to leave cells without phosphatase. Muscle cells do not contain phosphatase, so glucose 1-phosphate enters glycolysis and Krebs cycle. However, glycolysis produces lactic acid from glucose 1-phosphate which can be converted into glucose within the liver.
Glucose Anabolism: Gluconeogenesis
Glucose can be synthesized from protein and fat in a process known as gluconeogenesis. This occurs when glucose and glycogen are expended without reintroduction of carbohydrate foods. This process is normal within the body, but it does not happen at a mass scale unless starving, low consumption of carbs, or hormonal disorders.¹
Examples of amino acids used include cysteine, glycine, serine, threonine, and alanine. Lactic acid and glycerol - the backbone of triglycerides - are also used to synthesize glucose. Hormones, such as cortisol and glucagon, stimulate gluconeogenesis as well as thyroid hormones which mobilize proteins and fats to be used.¹ The following are steps describing the process of gluconeogenesis:
Certain amino acids and lactic acid are converted into pyruvic acid.
Pyruvic acid is converted into glyceraldehyde 3-phosphate; glycerol bypasses step one and converts into glyceraldehyde 3-phosphate.
Glyceraldehyde 3-phosphate is converted into glucose 6-phosphate.
Glucose 6-phosphate is converted into glucose.
Lipid Metabolism
After bile and pancreatic lipase break down fat to be absorbed in the small intestine, long-chain fatty acids, large short-chain fatty acids, and monoglycerides are transported via micelles into the absorptive intestinal cells. They are then rebuilt and packaged into chylomicrons, exiting the cell into lymph then systemic circulation. Once in adipose tissue, an apoprotein (identifying proteins on a chylomicron or other phospholipid) on the chylomicron known as apo C-2 activates endothelial lipoprotein lipase to remove fatty acids.¹ This is used for triglyceride synthesis within adipocytes (fat cells) or can be used for muscle ATP production.
Within the liver, very low-density lipoproteins (VLDLs) are formed in hepatocytes to transport endogenous (body-made) lipids to fat tissue. VLDLs are converted into low-density lipoproteins (LDLs) as triglycerides are removed. LDLs are composed of 50% cholesterol and responsible for transporting 75% of total blood cholesterol that binds to cells for cell structure, synthesis of bile salts, and steroid hormones.¹ Excessive LDLs deposit cholesterol within blood arteries, leading to diseases such as coronary heart disease - therefore being called ‘bad cholesterol’.
High-density lipoproteins (HDLs) remove cholesterol deposited by LDLs to the liver for elimination and are termed ‘good cholesterol’.
(INSERT PICS OF LPS)
Triglyceride Storage
Lipids that are not being used for ATP production are stored for future use within various parts of the body but mostly in subcutaneous tissue. Other areas include kidneys, muscles, genitals, etc.
Lipid Anabolism: Lipogenesis
Excess carbs and proteins are converted into fat for storage. Lipogenesis occurs when glucose and amino acids are converted into lipids. This is stimulated by insulin and occurs within the following pathways:
Lipogenesis: Glucose
Glucose ->glyceraldehyde 3-phosphate -> pyruvic acid -> acetyl coenzyme A -> fatty acids -> triglycerides (glycerol portion of triglycerides derived from glucose lipogenesis as well)
Glucose ->Glyceraldehyde 3-phosphate -> glycerol -> triglycerides (fatty acids portion of triglyceride derived from protein or glucose lipogenesis )
Lipogenesis: Protein
Certain amino acids -> acetyl coenzyme A -> fatty acids -> triglycerides (glycerol from glucose lipogenesis)
Resulting fatty acids can be stored or produce lipoproteins, phospholipids, and cholesterol.
Lipid Catabolism: Lipolysis
Lipid breakdown is known as lipolysis and is stimulated by stress hormones such as epinephrine, norepinephrine, and cortisol. Triglycerides are broken down into glycerol and fatty acids via different pathways. Fatty acids can be converted into ketone bodies via a process known as ketogenesis.
Glycerol:
Triglycerides -> glycerol -> glyceraldehyde 3-phosphate -> glucose (if ATP supply is high - known as gluconeogenesis)
Triglycerides -> glycerol -> glyceraldehyde 3-phosphate -> pyruvic acid (if ATP supply is low)
Fatty Acids:
Fatty Acids enter the Krebs cycle:
Triglycerides -> fatty acids ->* acetyl coenzyme A -> Krebs cycle
*beta-oxidation occurs
Ketogenesis
Fatty Acids turn into Ketones:
Triglycerides -> fatty acids ->* acetyl coenzyme A -> ketone bodies (acetoacetic acid, beta-hydroxybutyric acid, acetone)
*beta-oxidation occurs
Ketone bodies can convert into acetyl coenzyme A and enter the Krebs cycle. This is a normal part of lipid catabolism and blood levels of ketones are relatively low due to the fast conversion and uptake of acetoacetic acid into acetyl CoA.¹ Ketone bodies can also be converted into triglycerides if acetyl CoA form into fatty acids that are stored as triglycerides.
Ketone bodies-> acetyl CoA -> Krebs cycle
OR
Ketone bodies-> acetyl CoA ->* fatty acids -> triglycerides
*beta-oxidation occurs
Ketosis is when excess ketones are present above normal blood levels (due to conditions like diabetes mellitus; fatty meal consumption; few carbohydrate intake), and buffers such as sodium bicarbonate raises pH to counteract acidic ketone bodies. Chronic ketosis can lead to acidosis (low blood pH) that must be corrected medically.
Protein Metabolism
Once broken down into amino acids, proteins are not stored but rather synthesized into new proteins or ATP. Excess dietary amino acids are turned into glucose (gluconeogenesis) or triglycerides (lipogenesis). Proteins are used to function as enzymes, transporters (hemoglobin), clotting factors, hormones, and structures.
Protein Catabolism:
Protein breakdown is stimulated by cortisol and occurs normally to a certain extent. Proteins broken down into amino acids can be used to synthesize other amino acids, fatty acids, ketone bodies, or glucose.¹Amino acids must have the amino group removed (-NH3) before entering the Krebs cycle in a process called deamination; the amino group end product of ammonia is converted into urea by hepatocytes and excreted in the urine.
When protein is broken into fatty acids, it is known as lipogenesis. Protein converted into glucose is known as gluconeogenesis, and protein converted into ketone bodies is known as ketogenesis.¹
Protein Anabolism:
Protein anabolism involves the synthesis of peptide bonds between amino acids to create new proteins as instructed by DNA and RNA and stimulated by insulin-like growth factors (IGFs) thyroid hormones, insulin, and sex hormones.¹ Protein anabolism occurs by transcription and translation, as described in “Cells”. The body requires nonessential amino acids (10 amino acids the body can create adequate amounts) and essential amino acids (10 amino acids that must be provided by diet) for protein synthesis to occur.
Source(s):
1. Jerry Tortora and Bryan Dickerson, Principles of Anatomy & Physiology, 16th ed. (New Jersey: John Wiley & Sons, 2021).